Propagation of slow waves in the guinea-pig gastric antrum
- PMID: 16357017
- PMCID: PMC1805648
- DOI: 10.1113/jphysiol.2005.100735
Propagation of slow waves in the guinea-pig gastric antrum
Abstract
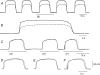
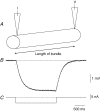
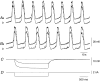
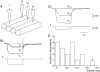
Intracellular recordings were made from the circular layer of the intact muscular wall of the guinea-pig gastric antrum in preparations where much of the corpus remained attached. When two electrodes were positioned parallel to and near to the greater curvature, slow waves were first detected at the oral site and subsequently at the anal site: the oro-anal conduction velocity was found to be 2.5 mm s(-1). When one electrode was positioned near the greater curvature and the other at a circumferential location, slow waves were first detected near the greater curvature and subsequently at the circumferential site: the circumferential conduction velocity was 13.9 mm s(-1). When recordings were made from preparations in which the circular muscle layer had been removed, the oro-anal and the circumferential conduction velocities were both about 3.5 mm s(-1). When slow waves were recorded from preparations in which much of the myenteric network of antral interstitial cells (ICC(MY)) had been dissected away, slow waves were first detected near the region of intact ICC(MY) and subsequently at a circumferential location: the circumferential conduction velocity of slow waves in regions devoid of ICC(MY) was 14.7 mm s(-1). When the electrical properties of isolated single bundles of circular muscle were determined, their length constants were about 3 mm and their time constant about 230 ms, giving an asymptotic electrotonic propagation velocity of 25 mm s(-1). Oro-anal electrical coupling between adjacent bundles of circular muscle was found to vary widely: some bundles were well connected to neighbouring bundles whereas others were not. Together the observations suggest that the slow oro-anal progression of slow waves results from a slow conduction velocity of pacemaker potentials in the myenteric network of interstitial cells. The rapid circumferential conduction of slow waves results from the electrical properties of the circular muscle layer which allow intramuscular ICC (ICC(IM)) to support the radial spread of slow waves: regions of high resistance between bundles prevent the anally directed spread of slow waves within the circular layer.
Figures





Comment in
-
All together now: from pacemakers to gastric peristalsis.J Physiol. 2006 Feb 15;571(Pt 1):1. doi: 10.1113/jphysiol.2005.104372. Epub 2006 Jan 12. J Physiol. 2006. PMID: 16410277 Free PMC article. No abstract available.
References
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit. Cell Tissue Res. 1997;290:11–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

