Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts
- PMID: 16357158
- PMCID: PMC2640458
- DOI: 10.1158/0008-5472.CAN-05-2211
Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts
Abstract
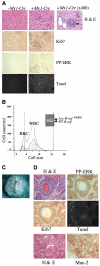
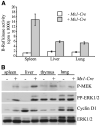
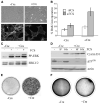
Mutations of the human B-RAF gene are detected in approximately 8% of cancer samples, primarily in cutaneous melanomas (70%). The most common mutation (90%) is a valine-to-glutamic acid mutation at residue 600 (V600E; formerly V599E according to previous nomenclature). Using a Cre/Lox approach, we have generated a conditional knock-in allele of (V600E)B-raf in mice. We show that widespread expression of (V600E)B-Raf cannot be tolerated in embryonic development, with embryos dying approximately 7.5 dpc. Directed expression of mutant (V600E)B-Raf to somatic tissues using the IFN-inducible Mx1-Cre mouse strain induces a proliferative disorder and bone marrow failure with evidence of nonlymphoid neoplasia of the histiocytic type leading to death within 4 weeks of age. However, expression of mutant B-Raf does not alter the proliferation profile of all somatic tissues. In primary mouse embryonic fibroblasts, expression of endogenous (V600E)B-Raf induces morphologic transformation, increased cell proliferation, and loss of contact inhibition. Thus, (V600E)B-Raf is able to induce several hallmarks of transformation in some primary mouse cells without evidence for the involvement of a cooperating oncogene or tumor suppressor gene.
Figures

References
-
- Jansen HW, Lurz R, Bister K, Bonner TI, Mark GE, Rapp UR. Homologous cell-derived oncogenes in avian carcinoma virus MH2 and murine sarcoma virus 3611. Nature. 1984;307:281–4. - PubMed
-
- Storm SM, Brennscheidt U, Sithanandam G, Rapp UR. raf oncogenes in carcinogenesis. Crit Rev Oncog. 1990;2:1–8. - PubMed
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

