The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor
- PMID: 16357220
- PMCID: PMC1315407
- DOI: 10.1101/gad.1373705
The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor
Abstract
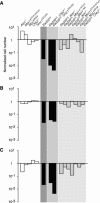
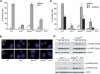
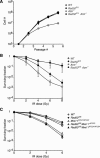
Genetic and cytologic data from Saccharomyces cerevisiae and mammals implicate the Mre11 complex, consisting of Mre11, Rad50, and Nbs1, as a sensor of DNA damage, and indicate that the complex influences the activity of ataxia-telangiectasia mutated (ATM) in the DNA damage response. Rad50(S/S) mice exhibit precipitous apoptotic attrition of hematopoietic cells. We generated ATM- and Chk2-deficient Rad50(S/S) mice and found that Rad50(S/S) cellular attrition was strongly ATM and Chk2 dependent. The hypomorphic Mre11(ATLD1) and Nbs1(Delta)(B) alleles conferred similar rescue of Rad50(S/S)-dependent hematopoietic failure. These data indicate that the Mre11 complex activates an ATM-Chk2-dependent apoptotic pathway. We find that apoptosis and cell cycle checkpoint activation are parallel outcomes of the Mre11 complex-ATM pathway. Conversely, the Rad50(S) mutation mitigated several phenotypic features of ATM deficiency. We propose that the Rad50(S) allele is hypermorphic for DNA damage signaling, and that the resulting constitutive low-level activation of the DNA damage response accounts for the partial suppression of ATM deficiency in Rad50(S/S) Atm(-/-) mice.
Figures



References
-
- Abraham R.T. 2004. PI 3-kinase related kinases: `Big' players in stress-induced signaling pathways. DNA Repair 3: 883-887. - PubMed
-
- Bakkenist C.J. and Kastan, M.B. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421: 499-506. - PubMed
-
- Barlow C., Hirotsune, S., Paylor, R., Liyanage, M., Eckhaus, M., Collins, F., Shiloh, Y., Crawley, J.N., Ried, T., Tagle, D., et al. 1996. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell 86: 159-171. - PubMed
-
- Bassing C.H., Suh, H., Ferguson, D.O., Chua, K.F., Manis, J., Eckersdorff, M., Gleason, M., Bronson, R., Lee, C., and Alt, F.W. 2003. Histone H2AX: A dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114: 359-370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
