Selective activation of TACI by syndecan-2
- PMID: 16357320
- PMCID: PMC1895754
- DOI: 10.1182/blood-2005-01-0256
Selective activation of TACI by syndecan-2
Abstract
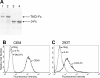
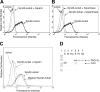
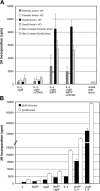
B-lymphocyte homeostasis and function are regulated by complementary actions of the TNFR family members TACI, BCMA, and BAFF-R, which are expressed by mature B cells. How these receptors are differentially activated is not entirely understood, because the primary ligand BAFF binds to all three. We searched for alternative ligands for TACI using recombinant TACI-Fc fusion protein as a probe and identified syndecan-2 as a new binding partner. TACI binding appears to require heparan sulfate posttranslational modifications of syndecan-2, because free heparin or pretreatment with heparitinase blocked the interaction. Syndecan-2 bound TACI but bound neither BAFF-R nor BCMA. Transfected cells expressing syndecan-2 activated signaling through TACI, as indicated by an NFAT-specific reporter. Syndecan-1 and syndecan-4 were also able to induce TACI signaling in a similar manner. This is the first identification of ligands that selectively activate TACI without simultaneously triggering BCMA or BAFF-R. This finding may help explain the alternative outcomes of signaling from this family of receptors in B cells.
Figures




References
-
- Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Ann Rev Immunol. 2003;21: 231-264. - PubMed
-
- Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27: 19-26. - PubMed
-
- Lentz VM, Cancro MP, Nashold FE, Hayes CE. Bcmd governs recruitment of new B cells into the stable peripheral B cell pool in the A/WySnJ mouse. J Immunol. 1996;157: 598-606. - PubMed
-
- Lentz VM, Hayes CE, Cancro MP. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J Immunol. 1998;160: 3743-3747. - PubMed
-
- Miller DJ, Hayes CE. Phenotypic and genetic characterization of a unique B lymphocyte deficiency in strain A/WySnJ mice. Eur J Immunol. 1991;21: 1123-1130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

