Virus membrane-fusion proteins: more than one way to make a hairpin
- PMID: 16357862
- PMCID: PMC7097298
- DOI: 10.1038/nrmicro1326
Virus membrane-fusion proteins: more than one way to make a hairpin
Abstract
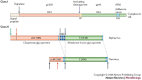
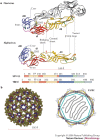
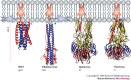
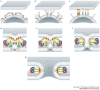
Structure-function studies have defined two classes of viral membrane-fusion proteins that have radically different architectures but adopt a similar overall 'hairpin' conformation to induce fusion of the viral and cellular membranes and therefore initiate infection. In both classes, the hairpin conformation is achieved after a conformational change is triggered by interaction with the target cell. This review will focus in particular on the properties of the more recently described class II proteins.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. - PubMed
-
- Sollner TH. Intracellular and viral membrane fusion: a uniting mechanism. Curr. Opin. Cell Biol. 2004;16:429–435. - PubMed
-
- Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. - PubMed
-
- Sieczkarski SB, Whittaker GR. Viral entry. Curr. Topics Microbiol. Immunol. 2005;285:1–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

