Intramuscular viral delivery of paraplegin rescues peripheral axonopathy in a model of hereditary spastic paraplegia
- PMID: 16357941
- PMCID: PMC1312020
- DOI: 10.1172/JCI26210
Intramuscular viral delivery of paraplegin rescues peripheral axonopathy in a model of hereditary spastic paraplegia
Erratum in
- J Clin Invest. 2014 Feb;124(2):871
Abstract
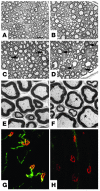
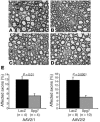
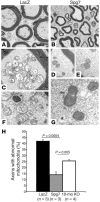
Degeneration of peripheral motor axons is a common feature of several debilitating diseases including complicated forms of hereditary spastic paraplegia. One such form is caused by loss of the mitochondrial energy-dependent protease paraplegin. Paraplegin-deficient mice display a progressive degeneration in several axonal tracts, characterized by the accumulation of morphological abnormal mitochondria. We show that adenoassociated virus-mediated (AAV-mediated) intramuscular delivery of paraplegin halted the progression of neuropathological changes and rescued mitochondrial morphology in the peripheral nerves of paraplegin-deficient mice. One single injection before onset of symptoms improved the motor performance of paraplegin-deficient mice for up to 10 months, indicating that the peripheral neuropathy contributes to the clinical phenotype. This study provides a proof of principle that gene transfer may be an effective therapeutic option for patients with paraplegin deficiency and demonstrates that AAV vectors can be successfully employed for retrograde delivery of an intracellular protein to spinal motor neurons, opening new perspectives for several hereditary axonal neuropathies of the peripheral nerves.
Figures

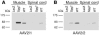
Similar articles
-
Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport.J Clin Invest. 2004 Jan;113(2):231-42. doi: 10.1172/JCI20138. J Clin Invest. 2004. PMID: 14722615 Free PMC article.
-
Alternative splicing of Spg7, a gene involved in hereditary spastic paraplegia, encodes a variant of paraplegin targeted to the endoplasmic reticulum.PLoS One. 2012;7(5):e36337. doi: 10.1371/journal.pone.0036337. Epub 2012 May 1. PLoS One. 2012. PMID: 22563492 Free PMC article.
-
SARM1 deletion delays cerebellar but not spinal cord degeneration in an enhanced mouse model of SPG7 deficiency.Brain. 2023 Oct 3;146(10):4117-4131. doi: 10.1093/brain/awad136. Brain. 2023. PMID: 37086482
-
Translating m-AAA protease function in mitochondria to hereditary spastic paraplegia.Trends Mol Med. 2006 Jun;12(6):262-9. doi: 10.1016/j.molmed.2006.04.002. Epub 2006 May 2. Trends Mol Med. 2006. PMID: 16647881 Review.
-
Molecular basis of inherited spastic paraplegias.Curr Opin Genet Dev. 2001 Jun;11(3):336-42. doi: 10.1016/s0959-437x(00)00199-4. Curr Opin Genet Dev. 2001. PMID: 11377972 Review.
Cited by
-
A spastic paraplegia mouse model reveals REEP1-dependent ER shaping.J Clin Invest. 2013 Oct;123(10):4273-82. doi: 10.1172/JCI65665. Epub 2013 Sep 24. J Clin Invest. 2013. PMID: 24051375 Free PMC article.
-
A Single Intravenous Injection of AAV-PHP.B-hNDUFS4 Ameliorates the Phenotype of Ndufs4 -/- Mice.Mol Ther Methods Clin Dev. 2020 May 4;17:1071-1078. doi: 10.1016/j.omtm.2020.04.026. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32478122 Free PMC article.
-
An Update on the Hereditary Spastic Paraplegias: New Genes and New Disease Models.Mov Disord Clin Pract. 2015 Jun 2;2(3):213-223. doi: 10.1002/mdc3.12184. eCollection 2015 Sep. Mov Disord Clin Pract. 2015. PMID: 30838228 Free PMC article. Review.
-
Insights into Clinical, Genetic, and Pathological Aspects of Hereditary Spastic Paraplegias: A Comprehensive Overview.Front Mol Biosci. 2021 Nov 26;8:690899. doi: 10.3389/fmolb.2021.690899. eCollection 2021. Front Mol Biosci. 2021. PMID: 34901147 Free PMC article. Review.
-
Pluripotent Stem Cells as a Preclinical Cellular Model for Studying Hereditary Spastic Paraplegias.Int J Mol Sci. 2024 Feb 23;25(5):2615. doi: 10.3390/ijms25052615. Int J Mol Sci. 2024. PMID: 38473862 Free PMC article. Review.
References
-
- Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151–1154. - PubMed
-
- Harding, A.E. 1984. Hereditary “pure” spastic paraplegia. In The hereditary ataxias and related disorders. Churchill Livingstone. New York, New York, USA. 174–191.
-
- Schwarz GA, Liu CN. Hereditary (familial) spastic paraplegia: further clinical and pathological observations. Arch. Neurol. Psychiatry. 1956;75:144–162. - PubMed
-
- Fink JK. Advances in the hereditary spastic paraplegias. Exp. Neurol. 2003;184(Suppl. 1):S106–S110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

