A new type of radiosensitive T-B-NK+ severe combined immunodeficiency caused by a LIG4 mutation
- PMID: 16357942
- PMCID: PMC1312018
- DOI: 10.1172/JCI26121
A new type of radiosensitive T-B-NK+ severe combined immunodeficiency caused by a LIG4 mutation
Abstract
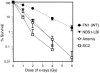
V(D)J recombination of Ig and TCR loci is a stepwise process during which site-specific DNA double-strand breaks (DSBs) are made by RAG1/RAG2, followed by DSB repair by nonhomologous end joining. Defects in V(D)J recombination result in SCID characterized by absence of mature B and T cells. A subset of T-B-NK+ SCID patients is sensitive to ionizing radiation, and the majority of these patients have mutations in Artemis. We present a patient with a new type of radiosensitive T-B-NK+ SCID with a defect in DNA ligase IV (LIG4). To date, LIG4 mutations have only been described in a radiosensitive leukemia patient and in 4 patients with a designated LIG4 syndrome, which is associated with chromosomal instability, pancytopenia, and developmental and growth delay. The patient described here shows that a LIG4 mutation can also cause T-B-NK+ SCID without developmental defects. The LIG4-deficient SCID patient had an incomplete but severe block in precursor B cell differentiation, resulting in extremely low levels of blood B cells. The residual D(H)-J(H) junctions showed extensive nucleotide deletions, apparently caused by prolonged exonuclease activity during the delayed D(H)-J(H) ligation process. In conclusion, different LIG4 mutations can result in either a developmental defect with minor immunological abnormalities or a SCID picture with normal development.
Figures




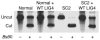
References
-
- Schatz DG. V(D)J recombination. Immunol. Rev. 2004;200:5–11. - PubMed
-
- Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu. Rev. Biochem. 2002;71:101–132. - PubMed
-
- McBlane JF, et al. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. - PubMed
-
- Van Gent DC, Ramsden DA, Gellert M. The RAG1 and RAG2 proteins establish the 12/23 rule in V(D)J recombination. Cell. 1996;85:107–113. - PubMed
-
- Lees-Miller SP, Meek K. Repair of DNA double strand breaks by non-homologous end joining. Biochimie. 2003;85:1161–1173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

