A selective role for MRF4 in innervated adult skeletal muscle: Na(V) 1.4 Na+ channel expression is reduced in MRF4-null mice
- PMID: 16358417
- PMCID: PMC6009121
- DOI: 10.3727/000000005783992034
A selective role for MRF4 in innervated adult skeletal muscle: Na(V) 1.4 Na+ channel expression is reduced in MRF4-null mice
Abstract
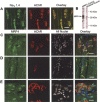
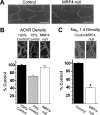
The factors that regulate transcription and spatial expression of the adult skeletal muscle Na+ channel, Na(V) 1.4, are poorly understood. Here we tested the role of the transcription factor MRF4, one of four basic helix-loop-helix (bHLH) factors expressed in skeletal muscle, in regulation of the Na(V) 1.4 Na+ channel. Overexpression of MRF4 in C2C12 muscle cells dramatically elevated Na(V) 1.4 reporter gene expression, indicating that MRF4 is more efficacious than the other bHLH factors expressed at high levels endogenously in these cells. In vivo, MRF4 protein was found both in extrajunctional and subsynaptic muscle nuclei. To test the importance of MRF4 in Na(V) 1.4 gene regulation in vivo, we examined Na+ channel expression in MRF4-null mice using several techniques, including Western blotting, immunocytochemistry, and electrophysiological recording. By all methods, we found that expression of the Na(V) 1.4 Na+ channel was substantially reduced in MRF4-null mice, both in the surface membrane and at neuromuscular junctions. In contrast, expression of the acetylcholine receptor, and in particular its alpha subunit, was unchanged, indicating that MRF4 regulation of Na+ channel expression was selective. Expression of the bHLH factors myf-5, MyoD, and myogenin was increased in MRF4-null mice, but these factors were not able to fully maintain Na(V) 1.4 Na+ channel expression either in the extrajunctional membrane or at the synapse. Thus, MRF4 appears to play a novel and selective role in adult muscle.
Figures





Similar articles
-
Denervation induces a rapid nuclear accumulation of MRF4 in mature myofibers.Dev Dyn. 2000 Jul;218(3):438-51. doi: 10.1002/1097-0177(200007)218:3<438::AID-DVDY1001>3.0.CO;2-6. Dev Dyn. 2000. PMID: 10878609
-
Overlapping functions of the myogenic bHLH genes MRF4 and MyoD revealed in double mutant mice.Development. 1998 Jul;125(13):2349-58. doi: 10.1242/dev.125.13.2349. Development. 1998. PMID: 9609818
-
Loss of synaptic vesicles from neuromuscular junctions in aged MRF4-null mice.Neuroreport. 2011 Mar 9;22(4):185-9. doi: 10.1097/WNR.0b013e328344493c. Neuroreport. 2011. PMID: 21278612 Free PMC article.
-
Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis.Semin Cell Dev Biol. 2017 Dec;72:19-32. doi: 10.1016/j.semcdb.2017.11.011. Epub 2017 Nov 15. Semin Cell Dev Biol. 2017. PMID: 29127046 Review.
-
The molecular basis of skeletal muscle differentiation.Semin Diagn Pathol. 1994 Feb;11(1):3-14. Semin Diagn Pathol. 1994. PMID: 8202645 Review.
Cited by
-
Transcription factors in muscle atrophy caused by blocked neuromuscular transmission and muscle unloading in rats.Mol Med. 2007 Sep-Oct;13(9-10):461-70. doi: 10.2119/2006-00066.Nordquist. Mol Med. 2007. PMID: 17622304 Free PMC article.
-
Promoting and accelerating muscle regeneration through cell therapy in a mouse model.J Taibah Univ Med Sci. 2024 Sep 21;19(5):1011-1023. doi: 10.1016/j.jtumed.2024.09.004. eCollection 2024 Oct. J Taibah Univ Med Sci. 2024. PMID: 39484055 Free PMC article.
-
Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals.J Mol Cell Cardiol. 2007 Nov;43(5):636-47. doi: 10.1016/j.yjmcc.2007.07.062. Epub 2007 Aug 10. J Mol Cell Cardiol. 2007. PMID: 17884088 Free PMC article.
-
Molecular differential expression of voltage-gated sodium channel α and β subunit mRNAs in five different mammalian cell lines.J Bioenerg Biomembr. 2011 Dec;43(6):729-38. doi: 10.1007/s10863-011-9399-7. Epub 2011 Nov 12. J Bioenerg Biomembr. 2011. PMID: 22081212
-
Divergent cis-regulatory evolution underlies the convergent loss of sodium channel expression in electric fish.Sci Adv. 2022 Jun 3;8(22):eabm2970. doi: 10.1126/sciadv.abm2970. Epub 2022 Jun 1. Sci Adv. 2022. PMID: 35648851 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
