Application of transmission electron microscopy to the clinical study of viral and bacterial infections: present and future
- PMID: 16361103
- PMCID: PMC7126980
- DOI: 10.1016/j.micron.2005.10.001
Application of transmission electron microscopy to the clinical study of viral and bacterial infections: present and future
Abstract
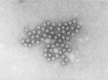
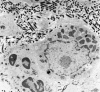
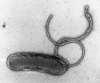
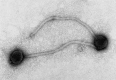
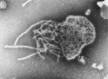
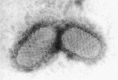
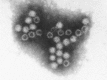
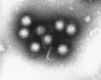
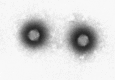
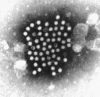
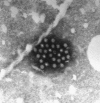
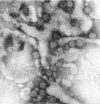
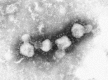
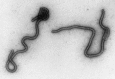
Transmission electron microscopy has had a profound impact on our knowledge and understanding of viruses and bacteria. The 1000-fold improvement in resolution provided by electron microscopy (EM) has allowed visualization of viruses, the existence of which had previously only been suspected as the causative agents of transmissible infectious disease. Viruses are grouped into families based on their morphology. Viruses from different families look different and these morphological variances are the basis for identification of viruses by EM. Electron microscopy initially came to prominence in diagnostic microbiology in the late 1960s when it was used in the rapid diagnosis of smallpox, by differentiating, on a morphological basis, poxviruses from the less problematic herpesviruses in skin lesions. Subsequently, the technique was employed in the diagnosis of other viral infections, such as hepatitis B and parvovirus B19. Electron microscopy has led to the discovery of many new viruses, most notably the various viruses associated with gastroenteritis, for which it remained the principal diagnostic method until fairly recent times. Development of molecular techniques, which offer greater sensitivity and often the capacity to easily process large numbers of samples, has replaced EM in many areas of diagnostic virology. Hence the role of EM in clinical virology is evolving with less emphasis on diagnosis and more on research, although this is likely only to be undertaken in specialist centres. However, EM still offers tremendous advantages to the microbiologist, both in the speed of diagnosis and the potential for detecting, by a single test, any viral pathogen or even multiple pathogens present within a sample. There is continuing use of EM for the investigation of new and emerging agents, such as SARS and human monkeypox virus. Furthermore, EM forms a vital part of the national emergency response programme of many countries and will provide a frontline diagnostic service in the event of a bioterrorism incident, particularly in the scenario of a deliberate release of smallpox virus. In the field of bacteriology, EM is of little use diagnostically, although some bacterial pathogens can be identified in biopsy material processed for EM examination. Electron microscopy has been used, however, to elucidate the structure and function of many bacterial features, such as flagellae, fimbriae and spores and in the study of bacteriophages. The combined use of EM and gold-labelled antibodies provides a powerful tool for the ultrastructural localisation of bacterial and viral antigens.
Figures

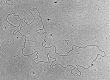
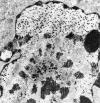
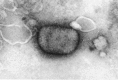
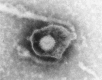

Similar articles
-
The role of electron microscopy in the rapid diagnosis of viral infections--review.Folia Microbiol (Praha). 2010 Jan;55(1):88-101. doi: 10.1007/s12223-010-0015-8. Epub 2010 Mar 25. Folia Microbiol (Praha). 2010. PMID: 20336511 Free PMC article. Review.
-
Electron microscopy: essentials for viral structure, morphogenesis and rapid diagnosis.Sci China Life Sci. 2013 May;56(5):421-30. doi: 10.1007/s11427-013-4476-2. Epub 2013 May 1. Sci China Life Sci. 2013. PMID: 23633074 Free PMC article. Review.
-
Rapid viral diagnosis: role of electron microscopy.New Microbiol. 2005 Jan;28(1):1-12. New Microbiol. 2005. PMID: 15782621 Review.
-
Virus detection by transmission electron microscopy: Still useful for diagnosis and a plus for biosafety.Rev Med Virol. 2019 Jan;29(1):e2019. doi: 10.1002/rmv.2019. Epub 2018 Nov 9. Rev Med Virol. 2019. PMID: 30411832 Free PMC article. Review.
-
Virus morphology as an aid for rapid diagnosis.Yale J Biol Med. 1980 Jan-Feb;53(1):19-25. Yale J Biol Med. 1980. PMID: 6155006 Free PMC article.
Cited by
-
Biosensors: frontiers in rapid detection of COVID-19.3 Biotech. 2020 Sep;10(9):385. doi: 10.1007/s13205-020-02369-0. Epub 2020 Aug 11. 3 Biotech. 2020. PMID: 32818132 Free PMC article. Review.
-
Real-time detection of virus antibody interaction by label-free common-path interferometry.Biophys Rep (N Y). 2023 Aug 2;3(3):100119. doi: 10.1016/j.bpr.2023.100119. eCollection 2023 Sep 13. Biophys Rep (N Y). 2023. PMID: 37662577 Free PMC article.
-
Proteomic analysis of the antimicrobial effects of sublethal concentrations of thymol on Salmonella enterica serovar Typhimurium.Appl Microbiol Biotechnol. 2020 Apr;104(8):3493-3505. doi: 10.1007/s00253-020-10390-9. Epub 2020 Feb 19. Appl Microbiol Biotechnol. 2020. PMID: 32072194
-
Systematic Comparison of Commercial Uranyl-Alternative Stains for Negative- and Positive-Staining Transmission Electron Microscopy of Organic Specimens.Adv Healthc Mater. 2025 Jun;14(16):e2404870. doi: 10.1002/adhm.202404870. Epub 2025 Apr 29. Adv Healthc Mater. 2025. PMID: 40302369 Free PMC article.
-
The role of electron microscopy in the rapid diagnosis of viral infections--review.Folia Microbiol (Praha). 2010 Jan;55(1):88-101. doi: 10.1007/s12223-010-0015-8. Epub 2010 Mar 25. Folia Microbiol (Praha). 2010. PMID: 20336511 Free PMC article. Review.
References
-
- Almeida J.D., Zuckerman A.J., Taylor P.E., Waterson A.P. Immune electron microscopy of the Australia-SH (serum hepatitis) antigen. Microbios. 1969;1:117–123.
-
- Almeida J.D., Rubenstein D., Stott E.J. New antigen-antibody system in Australia-antigen-positive hepatitis. Lancet. 1971;II:1225–1227. - PubMed
-
- Appleton H. Control of food-borne viruses. British Medical Bulletin. 2000;56:172–183. - PubMed
-
- Appleton H. Electron microscopy in virology. In: Crocker J., Burnett D., editors. The Science of Laboratory Diagnosis. second ed. Wiley; 2005. (Chapter 18)
-
- Appleton H., Higgins P.G. Viruses and gastroenteritis in infants. Lancet. 1975;I:1297. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

