Glc7-Reg1 phosphatase signals to Yck1,2 casein kinase 1 to regulate transport activity and glucose-induced inactivation of Saccharomyces maltose permease
- PMID: 16361229
- PMCID: PMC1456300
- DOI: 10.1534/genetics.105.051698
Glc7-Reg1 phosphatase signals to Yck1,2 casein kinase 1 to regulate transport activity and glucose-induced inactivation of Saccharomyces maltose permease
Abstract
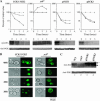
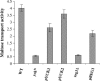
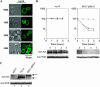
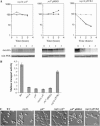
The Saccharomyces casein kinase 1 isoforms encoded by the essential gene pair YCK1 and YCK2 control cell growth and morphogenesis and are linked to the endocytosis of several membrane proteins. Here we define roles for the Yck1,2 kinases in Mal61p maltose permease activation and trafficking, using a yck1delta yck2-2(ts) (yck(ts)) strain with conditional Yck activity. Moreover, we provide evidence that Glc7-Reg1 phosphatase acts as an upstream activator of Yck1,2 kinases in a novel signaling pathway that modulates kinase activity in response to carbon source availability. The yck(ts) strain exhibits significantly reduced maltose transport activity despite apparently normal levels and cell surface localization of maltose permease protein. Glucose-induced internalization and rapid loss of maltose transport activity of Mal61/HAp-GFP are not observed in the yck(ts) strain and maltose permease proteolysis is blocked. We show that a reg1delta mutant exhibits a phenotype remarkably similar to that conferred by yck(ts). The reg1delta phenotype is not enhanced in the yck(ts) reg1delta double mutant and is suppressed by increased Yck1,2p dosage. Further, although Yck2p localization and abundance do not change in the reg1delta mutant, Yck1,2 kinase activity, as assayed by glucose-induced HXT1 expression and Mth1 repressor stability, is substantially reduced in the reg1delta strain.
Figures



Similar articles
-
Protein phosphatase type-1 regulatory subunits Reg1p and Reg2p act as signal transducers in the glucose-induced inactivation of maltose permease in Saccharomyces cerevisiae.Mol Gen Genet. 2000 Apr;263(3):411-22. doi: 10.1007/s004380051185. Mol Gen Genet. 2000. PMID: 10821175
-
Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I.Proc Natl Acad Sci U S A. 2004 Feb 10;101(6):1572-7. doi: 10.1073/pnas.0305901101. Epub 2004 Jan 30. Proc Natl Acad Sci U S A. 2004. PMID: 14755054 Free PMC article.
-
Sequences in the N-terminal cytoplasmic domain of Saccharomyces cerevisiae maltose permease are required for vacuolar degradation but not glucose-induced internalization.Curr Genet. 2006 Aug;50(2):101-14. doi: 10.1007/s00294-006-0080-3. Epub 2006 Jun 2. Curr Genet. 2006. PMID: 16741702
-
Yeast phospholipase C is required for stability of casein kinase I Yck2p and expression of hexose transporters.FEMS Microbiol Lett. 2017 Dec 1;364(22):fnx227. doi: 10.1093/femsle/fnx227. FEMS Microbiol Lett. 2017. PMID: 29087456 Free PMC article.
-
[Mechanism of ubiquitin homeostasis].Tanpakushitsu Kakusan Koso. 2010 Jan;55(1):55-60. Tanpakushitsu Kakusan Koso. 2010. PMID: 20058707 Review. Japanese. No abstract available.
Cited by
-
Role of casein kinase 1 in the glucose sensor-mediated signaling pathway in yeast.BMC Cell Biol. 2010 Mar 7;11:17. doi: 10.1186/1471-2121-11-17. BMC Cell Biol. 2010. PMID: 20205947 Free PMC article.
-
Gly-46 and His-50 of yeast maltose transporter Mal21p are essential for its resistance against glucose-induced degradation.J Biol Chem. 2009 Jun 5;284(23):15448-57. doi: 10.1074/jbc.M808151200. Epub 2009 Apr 7. J Biol Chem. 2009. PMID: 19359240 Free PMC article.
-
Regulation of Aspergillus nidulans CreA-Mediated Catabolite Repression by the F-Box Proteins Fbx23 and Fbx47.mBio. 2018 Jun 19;9(3):e00840-18. doi: 10.1128/mBio.00840-18. mBio. 2018. PMID: 29921666 Free PMC article.
-
Calcium Signaling Is a Universal Carbon Source Signal Transducer and Effects an Ionic Memory of Past Carbon Sources.Int J Mol Sci. 2025 Feb 28;26(5):2198. doi: 10.3390/ijms26052198. Int J Mol Sci. 2025. PMID: 40076822 Free PMC article.
-
A genomewide screen for tolerance to cationic drugs reveals genes important for potassium homeostasis in Saccharomyces cerevisiae.Eukaryot Cell. 2011 Sep;10(9):1241-50. doi: 10.1128/EC.05029-11. Epub 2011 Jul 1. Eukaryot Cell. 2011. PMID: 21724935 Free PMC article.
References
-
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerl and S. D. Emr, 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3: 271–282. - PubMed
-
- Babu, P., J. D. Bryan, H. R. Panek, S. L. Jordan, B. M. Forbrich et al., 2002. Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. J. Cell Sci. 115: 4957–4968. - PubMed
-
- Babu, P., R. J. Deschenes and L. C. Robinson, 2004. Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J. Biol. Chem. 279: 27138–27147. - PubMed
-
- Bonifacino, J. S., and L. M. Traub, 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72: 395–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

