Structural basis for conductance by the archaeal aquaporin AqpM at 1.68 A
- PMID: 16361443
- PMCID: PMC1323191
- DOI: 10.1073/pnas.0509469102
Structural basis for conductance by the archaeal aquaporin AqpM at 1.68 A
Abstract
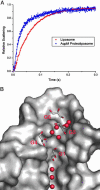
To explore the structural basis of the unique selectivity spectrum and conductance of the transmembrane channel protein AqpM from the archaeon Methanothermobacter marburgensis, we determined the structure of AqpM to 1.68-A resolution by x-ray crystallography. The structure establishes AqpM as being in a unique subdivision between the two major subdivisions of aquaporins, the water-selective aquaporins, and the water-plus-glycerol-conducting aquaglyceroporins. In AqpM, isoleucine replaces a key histidine residue found in the lumen of water channels, which becomes a glycine residue in aquaglyceroporins. As a result of this and other side-chain substituents in the walls of the channel, the channel is intermediate in size and exhibits differentially tuned electrostatics when compared with the other subfamilies.
Figures



References
-
- Preston, G. M., Carroll, T. P., Guggino, W. B. & Agre, P. (1992) Science 256, 385–387. - PubMed
-
- Fu, D., Libson, A., Miercke, L. J., Weitzman, C., Nollert, P., Krucinski, J. & Stroud, R. M. (2000) Science 290, 481–486. - PubMed
-
- Murata, K., Mitsuoka, K., Hirai, T., Walz, T., Agre, P., Heymann, J. B., Engel, A. & Fujiyoshi, Y. (2000) Nature 407, 599–605. - PubMed
-
- Sui, H., Han, B. G., Lee, J. K., Walian, P. & Jap, B. K. (2001) Nature 414, 872–878. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

