Disruption of Cryptochrome partially restores circadian rhythmicity to the arrhythmic period mutant of Drosophila
- PMID: 16361445
- PMCID: PMC1323156
- DOI: 10.1073/pnas.0505392102
Disruption of Cryptochrome partially restores circadian rhythmicity to the arrhythmic period mutant of Drosophila
Abstract
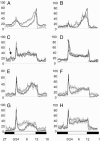
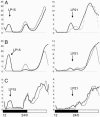
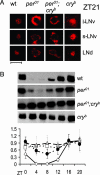
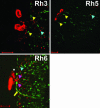
The Drosophila melanogaster circadian clock is generated by interlocked feedback loops, and null mutations in core genes such as period and timeless generate behavioral arrhythmicity in constant darkness. In light-dark cycles, the elevation in locomotor activity that usually anticipates the light on or off signals is severely compromised in these mutants. Light transduction pathways mediated by the rhodopsins and the dedicated circadian blue light photoreceptor cryptochrome are also critical in providing the circadian clock with entraining light signals from the environment. The cry(b) mutation reduces the light sensitivity of the fly's clock, yet locomotor activity rhythms in constant darkness or light-dark cycles are relatively normal, because the rhodopsins compensate for the lack of cryptochrome function. Remarkably, when we combined a period-null mutation with cry(b), circadian rhythmicity in locomotor behavior in light-dark cycles, as measured by a number of different criteria, was restored. This effect was significantly reduced in timeless-null mutant backgrounds. Circadian rhythmicity in constant darkness was not restored, and TIM protein did not exhibit oscillations in level or localize to the nuclei of brain neurons known to be essential for circadian locomotor activity. Therefore, we have uncovered residual rhythmicity in the absence of period gene function that may be mediated by a previously undescribed period-independent role for timeless in the Drosophila circadian pacemaker. Although we do not yet have a molecular correlate for these apparently iconoclastic observations, we provide a systems explanation for these results based on differential sensitivities of subsets of circadian pacemaker neurons to light.
Figures

References
-
- Stanewsky, R. (2003) J. Neurobiol. 54, 111-147. - PubMed
-
- Wheeler, D. A., Hamblen-Coyle, M. J., Dushay, M. S. & Hall, J. C. (1993) J. Biol. Rhythms 8, 67-94. - PubMed
-
- Emery, P., Stanewsky, R., Helfrich-Forster, C., Emery-Le, M., Hall, J. C. & Rosbash, M. (2000) Neuron 26, 493-504. - PubMed
-
- Helfrich-Förster, C., Winter, C., Hofbauer, A., Hall, J. C. & Stanewsky, R. (2001) Neuron 30, 249-261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

