Effect of repetitive administration of Doxorubicin-containing liposomes on plasma pharmacokinetics and drug biodistribution in a rat brain tumor model
- PMID: 16361575
- PMCID: PMC2883876
- DOI: 10.1158/1078-0432.CCR-05-1365
Effect of repetitive administration of Doxorubicin-containing liposomes on plasma pharmacokinetics and drug biodistribution in a rat brain tumor model
Abstract
Purpose: The incorporation of doxorubicin in long-circulating sterically stabilized liposomes (SSL-DXR) alters the pharmacokinetics and biodistribution of doxorubicin and therefore has the potential to alter the pharmacologic properties of doxorubicin. Previously, we showed that repetitive administration of SSL-DXR alters tumor vascular permeability.
Experimental design: Here, we investigated the effect of weekly i.v. injections of SSL-DXR on plasma pharmacokinetics and drug biodistribution in the orthotopic 9L rat brain tumor model.
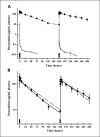
Results and conclusions: The pharmacokinetics of free doxorubicin (5.67 mg/kg) did not change with repeat dosing. In contrast, drug concentrations in plasma and brain tumor increased and deposition in liver and spleen decreased after administration of the second of two weekly doses of SSL-DXR. Noncompartmental analysis and descriptive pharmacokinetic models were created to test hypotheses relating to the mechanisms responsible for alterations in SSL-DXR deposition. The analysis suggested that weekly administration of SSL-DXR significantly (P < 0.05) decreased the plasma elimination rate of SSL-DXR (34%) and decreased drug deposition in liver (2-fold) and spleen (3.5-fold). The pharmacokinetic model that best captured the observed 2.5-fold increase in tumor uptake of SSL-DXR mediated by repeat dosing was one that hypothesized that the rates of drug influx/efflux into tumor were increased by the first dose of SSL-DXR. Models that accounted only for residual drug deposited in the tissue or blood by the first weekly injection provided inferior fits to the data. Thus, the effects of repetitive dosing on SSL-DXR deposition in tumor are consistent with a treatment-mediated alteration of tumor vascular permeability.
Figures


Similar articles
-
Mechanisms of tumor vascular priming by a nanoparticulate doxorubicin formulation.Pharm Res. 2012 Dec;29(12):3312-24. doi: 10.1007/s11095-012-0823-4. Epub 2012 Jul 14. Pharm Res. 2012. PMID: 22798260 Free PMC article.
-
Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer.Biochim Biophys Acta. 2004 May 27;1663(1-2):167-77. doi: 10.1016/j.bbamem.2004.03.006. Biochim Biophys Acta. 2004. PMID: 15157619
-
Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection.J Control Release. 2006 Oct 27;115(3):251-8. doi: 10.1016/j.jconrel.2006.08.017. Epub 2006 Sep 3. J Control Release. 2006. PMID: 17045355
-
Antivascular and antitumor activities of liposome-associated drugs.Anticancer Res. 2004 Mar-Apr;24(2A):397-404. Anticancer Res. 2004. PMID: 15152936 Review.
-
In vitro tests to predict in vivo performance of liposomal dosage forms.Chem Phys Lipids. 1993 Sep;64(1-3):219-37. doi: 10.1016/0009-3084(93)90067-d. Chem Phys Lipids. 1993. PMID: 8242835 Review.
Cited by
-
Vascular normalization in orthotopic glioblastoma following intravenous treatment with lipid-based nanoparticulate formulations of irinotecan (Irinophore C™), doxorubicin (Caelyx®) or vincristine.BMC Cancer. 2011 Apr 8;11:124. doi: 10.1186/1471-2407-11-124. BMC Cancer. 2011. PMID: 21477311 Free PMC article.
-
A Multiscale Physiologically-Based Pharmacokinetic Model for Doxorubicin to Explore its Mechanisms of Cytotoxicity and Cardiotoxicity in Human Physiological Contexts.Pharm Res. 2018 Jul 9;35(9):174. doi: 10.1007/s11095-018-2456-8. Pharm Res. 2018. PMID: 29987398 Free PMC article.
-
Evidence for distinct mechanisms of uptake and antitumor activity of secretory phospholipase A2 responsive liposome in prostate cancer.Integr Biol (Camb). 2013 Jan;5(1):172-82. doi: 10.1039/c2ib20108a. Integr Biol (Camb). 2013. PMID: 22890797 Free PMC article.
-
Novel drug-delivery approaches to the blood-brain barrier.Neurosci Bull. 2015 Apr;31(2):257-64. doi: 10.1007/s12264-014-1498-0. Epub 2015 Jan 16. Neurosci Bull. 2015. PMID: 25595370 Free PMC article. Review.
-
Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model.J Control Release. 2013 Jul 10;169(1-2):103-11. doi: 10.1016/j.jconrel.2013.04.007. Epub 2013 Apr 18. J Control Release. 2013. PMID: 23603615 Free PMC article.
References
-
- Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9:253–66. - PubMed
-
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. - PubMed
-
- Gabizon A, Shiota R, Papahadjopoulos D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J Natl Cancer Inst. 1989;81:1484–8. - PubMed
-
- Siegal T, Horowitz A, Gabizon A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: biodistribution and therapeutic efficacy. J Neurosurg. 1995;83:1029–37. - PubMed
-
- Sharma US, Sharma A, Chau RI, Straubinger RM. Liposome-mediated therapy of intracranial brain tumors in a rat model. Pharm Res. 1997;14:992–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

