The long term retention of levetiracetam in a large cohort of patients with epilepsy
- PMID: 16361605
- PMCID: PMC2117408
- DOI: 10.1136/jnnp.2005.064626
The long term retention of levetiracetam in a large cohort of patients with epilepsy
Abstract
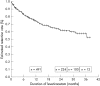
Levetiracetam (Lev) is a new antiepileptic drug with a distinct mechanism of action, shown in regulatory trials to be effective. These controlled trials do not always predict how useful a drug will be in day to day clinical practice. Retention rates can provide a better indication of efficacy and tolerability in everyday use. Patients attending a tertiary referral centre for epilepsy and who received Lev in the first 2 years of its marketing were assessed (n = 811) to determine continuation rates of treatment with this drug. At the last follow up, 65% of patients were still taking Lev, and the estimated 3 year retention rate was 58%. In total, 11% attained seizure freedom of at least 6 months. Patients taking greater numbers of concurrent antiepileptic drugs (AEDs) were more likely to discontinue Lev, and those reaching higher maximum daily dosages were less likely to discontinue Lev. The retention rate for Lev compares favourably with that of other new AEDs.
Conflict of interest statement
Competing interests: our department has received research and travel grants from UCB Pharma, the manufacturer of levetiracetam. M J Koepp, J S Duncan and J W Sander have received honoraria for speaking at meetings sponsored by UCB. J W Sander was a paid member of an advisory panel to UCB.
References
-
- Walker M C, Sander J W. The impact of new antiepileptic drugs on the prognosis of epilepsy: seizure freedom should be the ultimate goal. Neurology 199646912–914. - PubMed
-
- Walker M C, Sander J W. Developments in antiepileptic drug therapy. Curr Opin Neurol 19947131–139. - PubMed
-
- Kaplan E L, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Ass 195853457–481.