Predictive utility of circulating methylated DNA in serum of melanoma patients receiving biochemotherapy
- PMID: 16361635
- PMCID: PMC2856438
- DOI: 10.1200/JCO.2005.02.9876
Predictive utility of circulating methylated DNA in serum of melanoma patients receiving biochemotherapy
Abstract
Purpose: Currently, no validated blood-based assays accurately predict treatment response or outcome in melanoma patients. We hypothesized that methylation of tumor-related genes detected in serum DNA could predict disease outcome and therapeutic response in patients receiving concurrent biochemotherapy (BC) for metastatic melanoma.
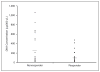
Patients and methods: American Joint Committee on Cancer stage IV melanoma patients (N = 50) had blood drawn before administration of BC. Patients (n = 47) were classified as BC responders or nonresponders. Responders (n = 23) demonstrated a complete or partial response following BC; nonresponders (n = 24) demonstrated progressive disease. Hypermethylation of Ras association domain family 1 (RASSF1A), retinoic acid receptor-beta2 (RAR-beta2), and O6-methylguanine DNA methyltransferase (MGMT) genes were assessed by methylation-specific polymerase chain reaction.
Results: Circulating methylated RASSF1A was significantly less frequent for responders (three of 23 patients; 13%) than nonresponders (10 of 24 patients; 42%; P = .028). Patients with RASSF1A, RAR-beta2, or at least one serum methylated gene had significantly worse overall survival than patients with no methylated genes (log-rank, P = .013, .021, and .01, respectively). Methylated RASSF1A was the only factor that significantly correlated with overall survival and BC response (risk ratio, 2.38; 95% CI, 1.16 to 4.86; P = .018; odds ratio = 0.21; 95% CI, 0.05 to 0.90; P = .036).
Conclusion: Detection of circulating methylated DNA in serum can predict response to BC and disease outcome.
Conflict of interest statement
Although all authors completed the disclosure declaration, the following author or immediate family members indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
[Table: see text]
Figures

Similar articles
-
Serial monitoring of circulating melanoma cells during neoadjuvant biochemotherapy for stage III melanoma: outcome prediction in a multicenter trial.J Clin Oncol. 2005 Nov 1;23(31):8057-64. doi: 10.1200/JCO.2005.02.0958. J Clin Oncol. 2005. PMID: 16258104 Free PMC article.
-
Treatment of metastatic melanoma with combined chemotherapy containing cisplatin, vinblastine and dacarbazine (CVD) and biotherapy using interleukin-2 and interferon-alpha.Ann Oncol. 1996 Oct;7(8):827-35. doi: 10.1093/oxfordjournals.annonc.a010762. Ann Oncol. 1996. PMID: 8922197 Clinical Trial.
-
Biochemotherapy in patients with advanced vulvovaginal mucosal melanoma.Melanoma Res. 2004 Dec;14(6):517-20. doi: 10.1097/00008390-200412000-00012. Melanoma Res. 2004. PMID: 15577323
-
Promoter methylation as biomarkers for diagnosis of melanoma: A systematic review and meta-analysis.J Cell Physiol. 2019 May;234(5):7356-7367. doi: 10.1002/jcp.27495. Epub 2018 Oct 28. J Cell Physiol. 2019. PMID: 30370527
-
[Treatment of metastasized malignant melanoma].Praxis (Bern 1994). 2001 Mar 8;90(10):391-6. Praxis (Bern 1994). 2001. PMID: 11305184 Review. German.
Cited by
-
RASSF1A hypermethylation in pretreatment serum DNA of neuroblastoma patients: a prognostic marker.Br J Cancer. 2009 Jan 27;100(2):399-404. doi: 10.1038/sj.bjc.6604887. Br J Cancer. 2009. PMID: 19165202 Free PMC article.
-
Alu hypomethylation and MGMT hypermethylation in serum as biomarkers of glioma.Oncotarget. 2017 Aug 7;8(44):76797-76806. doi: 10.18632/oncotarget.20012. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100349 Free PMC article.
-
CpG island hypermethylation profile in the serum of men with clinically localized and hormone refractory metastatic prostate cancer.J Urol. 2008 Feb;179(2):529-34; discussion 534-5. doi: 10.1016/j.juro.2007.09.038. J Urol. 2008. PMID: 18076941 Free PMC article.
-
Methylome analysis identifies a Wilms tumor epigenetic biomarker detectable in blood.Genome Biol. 2014 Aug 19;15(8):434. doi: 10.1186/s13059-014-0434-y. Genome Biol. 2014. PMID: 25134821 Free PMC article.
-
DNA methylation and gene deletion analysis of brain metastases in melanoma patients identifies mutually exclusive molecular alterations.Neuro Oncol. 2014 Nov;16(11):1499-509. doi: 10.1093/neuonc/nou107. Epub 2014 Jun 26. Neuro Oncol. 2014. PMID: 24968695 Free PMC article.
References
-
- Greene FL, Fleming ID, Fritz AG, et al. AJCC Cancer Staging Manual. 6. New York, NY: Springer-Verlag; 2002. pp. 229–254.
-
- O’Day SJ, Boasberg PD, Piro L, et al. Maintenance biotherapy for metastatic melanoma with interleukin-2 and granulocyte macrophage-colony stimulating factor improves survival for patients responding to induction concurrent biochemotherapy. Clin Cancer Res. 2002;8:2775–2781. - PubMed
-
- O’Day SJ, Gammon G, Boasberg PD, et al. Advantages of concurrent biochemotherapy modified by decrescendo interleukin-2, granulocyte colony-stimulating factor, and tamoxifen for patients with metastatic melanoma. J Clin Oncol. 1999;17:2752–2761. - PubMed
-
- Gollob JA, Veenstra KG, Parker RA, et al. Phase I trial of concurrent twice-weekly recombinant human interleukin-12 plus low-dose IL-2 in patients with melanoma or renal cell carcinoma. J Clin Oncol. 2003;21:2564–2573. - PubMed
-
- O’Day SJ, Atkins MB, Weber J. A phase II multi-center trial of maintenance biotherapy (MBT) after induction concurrent biochemotherapy (BCT) for patients (Pts) with metastatic melanoma. J Clin Oncol. 23(suppl; abstr 7503):710s, 205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

