Huckebein-mediated autoregulation of Glide/Gcm triggers glia specification
- PMID: 16362045
- PMCID: PMC1356350
- DOI: 10.1038/sj.emboj.7600907
Huckebein-mediated autoregulation of Glide/Gcm triggers glia specification
Abstract
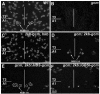
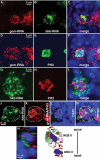
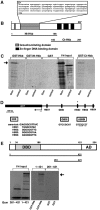
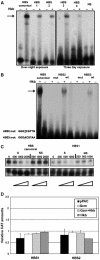
Cell specification in the nervous system requires patterning genes dictating spatio-temporal coordinates as well as fate determinants. In the case of neurons, which are controlled by the family of proneural transcription factors, binding specificity and patterned expression trigger both differentiation and specification. In contrast, a single gene, glide cell deficient/glial cell missing (glide/gcm), is sufficient for all fly lateral glial differentiation. How can different types of cells develop in the presence of a single fate determinant, that is, how do differentiation and specification pathways integrate and produce distinct glial populations is not known. By following an identified lineage, we here show that glia specification is triggered by high glide/gcm expression levels, mediated by cell-specific protein-protein interactions. Huckebein (Hkb), a lineage-specific factor, provides a molecular link between glide/gcm and positional cues. Importantly, Hkb does not activate transcription; rather, it physically interacts with Glide/Gcm thereby triggering its autoregulation. These data emphasize the importance of fate determinant cell-specific quantitative regulation in the establishment of cell diversity.
Figures


Similar articles
-
A novel role of the glial fate determinant glial cells missing in hematopoiesis.Int J Dev Biol. 2009;53(7):1013-22. doi: 10.1387/ijdb.082726cj. Int J Dev Biol. 2009. PMID: 19598118
-
The Repo Homeodomain Transcription Factor Suppresses Hematopoiesis in Drosophila and Preserves the Glial Fate.J Neurosci. 2019 Jan 9;39(2):238-255. doi: 10.1523/JNEUROSCI.1059-18.2018. Epub 2018 Nov 30. J Neurosci. 2019. PMID: 30504274 Free PMC article.
-
Positive autoregulation of the glial promoting factor glide/gcm.EMBO J. 1998 Nov 2;17(21):6316-26. doi: 10.1093/emboj/17.21.6316. EMBO J. 1998. PMID: 9799239 Free PMC article.
-
Evolutionary Conservation of the Gcm/Glide Cascade: Of Glia and Beyond.Brain Behav Evol. 2025;100(1):58-66. doi: 10.1159/000542753. Epub 2024 Nov 25. Brain Behav Evol. 2025. PMID: 39586239 Review.
-
glide/gcm: at the crossroads between neurons and glia.Curr Opin Genet Dev. 2002 Aug;12(4):465-72. doi: 10.1016/s0959-437x(02)00327-1. Curr Opin Genet Dev. 2002. PMID: 12100894 Review.
Cited by
-
Cis-regulatory logic driving glial cells missing: self-sustaining circuitry in later embryogenesis.Dev Biol. 2012 Apr 15;364(2):259-67. doi: 10.1016/j.ydbio.2012.02.003. Dev Biol. 2012. PMID: 22509525 Free PMC article.
-
GRG5/AES interacts with T-cell factor 4 (TCF4) and downregulates Wnt signaling in human cells and zebrafish embryos.PLoS One. 2013 Jul 1;8(7):e67694. doi: 10.1371/journal.pone.0067694. Print 2013. PLoS One. 2013. PMID: 23840876 Free PMC article.
-
SoxNeuro orchestrates central nervous system specification and differentiation in Drosophila and is only partially redundant with Dichaete.Genome Biol. 2014 May 30;15(5):R74. doi: 10.1186/gb-2014-15-5-r74. Genome Biol. 2014. PMID: 24886562 Free PMC article.
-
hkb is required for DIP-α expression and target recognition in the Drosophila neuromuscular circuit.Commun Biol. 2024 Apr 27;7(1):507. doi: 10.1038/s42003-024-06184-8. Commun Biol. 2024. PMID: 38678127 Free PMC article.
-
Coordination of cell cycle and morphogenesis during organ formation.Elife. 2024 Jan 26;13:e95830. doi: 10.7554/eLife.95830. Elife. 2024. PMID: 38275142 Free PMC article.
References
-
- Akiyama-Oda Y, Hosoya T, Hotta Y (1998) Alteration of cell fate by ectopic expression of Drosophila glial cells missing in non-neural cells. Dev Genes Evol 208: 578–585 - PubMed
-
- Akiyama-Oda Y, Hosoya T, Hotta Y (1999) Asymmetric cell division of thoracic neuroblast 6–4 to bifurcate glial and neuronal lineage in Drosophila. Development 126: 1967–1974 - PubMed
-
- Allan DW, Park D, St Pierre SE, Taghert PH, Thor S (2005) Regulators acting in combinatorial codes also act independently in single differentiating neurons. Neuron 45: 689–700 - PubMed
-
- Allan DW, Thor S (2003) Together at last: bHLH and LIM-HD regulators cooperate to specify motor neurons. Neuron 38: 675–677 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

