Characterization of IL-2-activated TILs and their use in intrapericardial immunotherapy in malignant pericardial effusion
- PMID: 16362409
- PMCID: PMC11029824
- DOI: 10.1007/s00262-005-0112-8
Characterization of IL-2-activated TILs and their use in intrapericardial immunotherapy in malignant pericardial effusion
Abstract
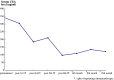
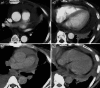
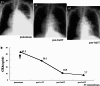
Pericardial effusion (PE) and cardiac tamponade caused by malignant pericarditis are critical conditions in cancer patients, which still lack a recommended protocol for their long-term management. Percutaneous pericardiocentesis and simple drainage are commonly performed as the initial treatment. The aims of this study were to investigate the presence of cytotoxic T lymphocytes (CTLs) in malignant PE and to determine the clinical response to administering autologous tumor-infiltrating lymphocytes (TILs) into the pericardial cavity. Initially, we identified human lymphocyte antigen class-I-restricted and tumor-specific CTLs within the interleukin-2 (IL-2)-activated TILs in PEs from four patients, on the basis of interferon-gamma production and lactate dehydrogenase-release assays. Clinically we observed favorable responses to the pericardial transfer of IL-2-activated autologous TILs in four patients: one male with advanced esophageal cancer, one female with recurrent lung cancer and two females with recurrent breast cancer, respectively. Autologous TILs from PEs were expanded in vitro with IL-2, characterized for CD3, CD4 and CD8 markers, checked for contamination and then infused into the patient's pericardial space through a catheter. This was repeated biweekly. After treatment, there were no signs of recurrence of PE in either case, as determined by radiography, echocardiography and computed tomography. The only adverse effects seen were grade 1 fevers. These results suggested that intrapericardial cellular immunotherapy with autologous TILs could be a safe and effective treatment for controlling malignant pericarditis with associated cardiac tamponade, and that tumor-specific CTLs present in malignant PE might be important for tumor rejection.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

