How the SARS coronavirus causes disease: host or organism?
- PMID: 16362992
- PMCID: PMC7168100
- DOI: 10.1002/path.1897
How the SARS coronavirus causes disease: host or organism?
Abstract
The previous epidemic of severe acute respiratory syndrome (SARS) has ended. However, many questions concerning how the aetiological agent, the novel SARS coronavirus (CoV), causes illness in humans remain unanswered. The pathology of fatal cases of SARS is dominated by diffuse alveolar damage. Specific histological changes are not detected in other organs. These contrast remarkably with the clinical picture, in which there are apparent manifestations in multiple organs. Both pathogen and host factors are important in the pathogenesis of SARS. The choice of specific receptors and the unique genome of the SARS-CoV are important elements in understanding the biology of the pathogen. For the host cells, the outcome of SARS-CoV infection, whether there are cytopathic effects or not, depends on the cell types that are infected. At the whole-body level, immune-mediated damage, due to activation of cytokines and/or chemokines and, perhaps, autoimmunity, may play key roles in the clinical and pathological features of SARS. Continued research is still required to determine the pathogenetic mechanisms involved and to combat this new emerging human infectious disease.
Copyright 2006 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures
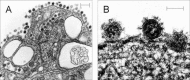
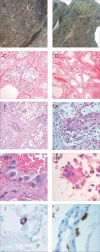
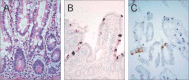
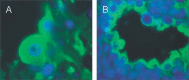
Similar articles
-
Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis.mBio. 2018 Oct 9;9(5):e01753-18. doi: 10.1128/mBio.01753-18. mBio. 2018. PMID: 30301856 Free PMC article.
-
Pathogenesis of severe acute respiratory syndrome.Curr Opin Immunol. 2005 Aug;17(4):404-10. doi: 10.1016/j.coi.2005.05.009. Curr Opin Immunol. 2005. PMID: 15950449 Free PMC article. Review.
-
Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus.Am J Respir Cell Mol Biol. 2013 Jun;48(6):742-8. doi: 10.1165/rcmb.2012-0339OC. Am J Respir Cell Mol Biol. 2013. PMID: 23418343 Free PMC article.
-
Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2).J Pathol. 2004 Jul;203(3):740-3. doi: 10.1002/path.1597. J Pathol. 2004. PMID: 15221932 Free PMC article.
-
Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus.Cell Mol Life Sci. 2004 Nov;61(21):2738-43. doi: 10.1007/s00018-004-4242-5. Cell Mol Life Sci. 2004. PMID: 15549175 Free PMC article. Review.
Cited by
-
Hypercytokinemia and Pathogen-Host Interaction in COVID-19.J Inflamm Res. 2020 Jun 23;13:255-261. doi: 10.2147/JIR.S259096. eCollection 2020. J Inflamm Res. 2020. PMID: 32606886 Free PMC article.
-
Down-regulation of granulocyte-macrophage colony-stimulating factor by 3C-like proteinase in transfected A549 human lung carcinoma cells.BMC Immunol. 2011 Feb 17;12:16. doi: 10.1186/1471-2172-12-16. BMC Immunol. 2011. PMID: 21324206 Free PMC article.
-
IILLS: predicting virus-receptor interactions based on similarity and semi-supervised learning.BMC Bioinformatics. 2019 Dec 27;20(Suppl 23):651. doi: 10.1186/s12859-019-3278-3. BMC Bioinformatics. 2019. PMID: 31881820 Free PMC article.
-
Diagnostics of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid antigen using chicken immunoglobulin Y.Poult Sci. 2012 Mar;91(3):636-42. doi: 10.3382/ps.2011-01916. Poult Sci. 2012. PMID: 22334738 Free PMC article.
-
Annexin A2 on lung epithelial cell surface is recognized by severe acute respiratory syndrome-associated coronavirus spike domain 2 antibodies.Mol Immunol. 2010 Feb;47(5):1000-9. doi: 10.1016/j.molimm.2009.11.019. Epub 2009 Dec 16. Mol Immunol. 2010. PMID: 20015551 Free PMC article.
References
-
- Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003; 348: 1986–1994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

