Ca2+ release triggered by NAADP in hepatocyte microsomes
- PMID: 16363992
- PMCID: PMC1422766
- DOI: 10.1042/BJ20051002
Ca2+ release triggered by NAADP in hepatocyte microsomes
Abstract
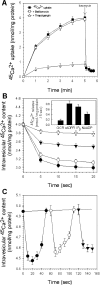
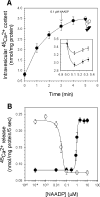
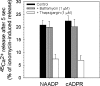
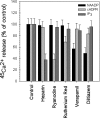
NAADP (nicotinic acid-adenine dinucleotide phosphate) is fast emerging as a new intracellular Ca2+-mobilizing messenger. NAADP induces Ca2+ release by a mechanism that is distinct from IP3 (inositol 1,4,5-trisphosphate)- and cADPR (cADP-ribose)-induced Ca2+ release. In the present study, we demonstrated that micromolar concentrations of NAADP trigger Ca2+ release from rat hepatocyte microsomes. Cross-desensitization to IP3 and cADPR by NAADP did not occur in liver microsomes. We report that non-activating concentrations of NAADP can fully inactivate the NAADP-sensitive Ca2+-release mechanism in hepatocyte microsomes. The ability of thapsigargin to block the NAADP-sensitive Ca2+ release is not observed in sea-urchin eggs or in intact mammalian cells. In contrast with the Ca2+ release induced by IP3 and cADPR, the Ca2+ release induced by NAADP was completely independent of the free extravesicular Ca2+ concentration and pH (in the range 6.4-7.8). The NAADP-elicited Ca2+ release cannot be blocked by the inhibitors of the IP3 receptors and the ryanodine receptor. On the other hand, verapamil and diltiazem do inhibit the NAADP- (but not IP3- or cADPR-) induced Ca2+ release.
Figures


Similar articles
-
NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores.EMBO J. 2001 Jun 1;20(11):2666-71. doi: 10.1093/emboj/20.11.2666. EMBO J. 2001. PMID: 11387201 Free PMC article.
-
NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors.J Cell Biol. 2003 Oct 27;163(2):271-82. doi: 10.1083/jcb.200306134. Epub 2003 Oct 20. J Cell Biol. 2003. PMID: 14568993 Free PMC article.
-
Activation and inactivation of Ca2+ release by NAADP+.J Biol Chem. 1996 Apr 12;271(15):8513-6. doi: 10.1074/jbc.271.15.8513. J Biol Chem. 1996. PMID: 8621471
-
NAADP-induced calcium release in sea urchin eggs.Biol Cell. 2000 Jul;92(3-4):197-204. doi: 10.1016/s0248-4900(00)01070-4. Biol Cell. 2000. PMID: 11043408 Review.
-
NAADP receptors.Cell Calcium. 2005 Sep-Oct;38(3-4):273-80. doi: 10.1016/j.ceca.2005.06.031. Cell Calcium. 2005. PMID: 16111747 Review.
Cited by
-
Calcium signaling in airway smooth muscle.Proc Am Thorac Soc. 2008 Jan 1;5(1):15-22. doi: 10.1513/pats.200704-047VS. Proc Am Thorac Soc. 2008. PMID: 18094080 Free PMC article. Review.
-
The luminal Ca(2+) chelator, TPEN, inhibits NAADP-induced Ca(2+) release.Cell Calcium. 2012 Dec;52(6):481-7. doi: 10.1016/j.ceca.2012.09.001. Epub 2012 Oct 23. Cell Calcium. 2012. PMID: 23099186 Free PMC article.
-
Mechanisms of carvedilol-induced [Ca2+] i rises and death in human hepatoma cells.Naunyn Schmiedebergs Arch Pharmacol. 2007 Nov;376(3):185-94. doi: 10.1007/s00210-007-0191-5. Epub 2007 Oct 5. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17917717
-
Pyridine nucleotide regulation of hepatic endoplasmic reticulum calcium uptake.Physiol Rep. 2019 Jul;7(12):e14151. doi: 10.14814/phy2.14151. Physiol Rep. 2019. PMID: 31222964 Free PMC article.
-
NAADP receptors.Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a004036. doi: 10.1101/cshperspect.a004036. Cold Spring Harb Perspect Biol. 2011. PMID: 21047915 Free PMC article. Review.
References
-
- Berridge M. J. Cell signalling: a tale of two messengers. Nature (London) 1993;361:315–325. - PubMed
-
- Lee H. C. Calcium signalling: NAADP ascends as a new messenger. Curr. Biol. 2003;13:R186–R188. - PubMed
-
- Patel S., Joseph S. K., Thomas A. P. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. - PubMed
-
- Lee H. C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001;41:317–345. - PubMed
-
- Berridge M. J., Lipp P., Bootman M. D. The versatility and universatility of calcium signalling. Nature Rev. Mol. Cell Biol. 2001;1:11–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

