Safer injections following a new national medicine policy in the public sector, Burkina Faso 1995-2000
- PMID: 16364178
- PMCID: PMC1343564
- DOI: 10.1186/1471-2458-5-136
Safer injections following a new national medicine policy in the public sector, Burkina Faso 1995-2000
Abstract
Background: The common failure of health systems to ensure adequate and sufficient supplies of injection devices may have a negative impact on injection safety. We conducted an assessment in April 2001 to determine to which extent an increase in safe injection practices between 1995 and 2000 was related to the increased access to injection devices because of a new essential medicine policy in Burkina Faso.
Methods: We reviewed outcomes of the new medicine policy implemented in 1995. In April 2001, a retrospective programme review assessed the situation between 1995 and 2000. We visited 52 health care facilities where injections had been observed during a 2000 injection safety assessment and their adjacent operational public pharmaceutical depots. Data collection included structured observations of available injection devices and an estimation of the proportion of prescriptions including at least one injection. We interviewed wholesaler managers at national and regional levels on supply of injection devices to public health facilities.
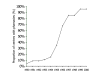
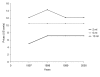
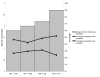
Results: Fifty of 52 (96%) health care facilities were equipped with a pharmaceutical depot selling syringes and needles, 37 (74%) of which had been established between 1995 and 2000. Of 50 pharmaceutical depots, 96% had single-use 5 ml syringes available. At all facilities, patients were buying syringes and needles out of the depot for their injections prescribed at the dispensary. While injection devices were available in greater quantities, the proportion of prescriptions including at least one injection remained stable between 1995 (26.5%) and 2000 (23.8%).
Conclusion: The implementation of pharmaceutical depots next to public health care facilities increased geographical access to essential medicines and basic supplies, among which syringes and needles, contributing substantially to safer injection practices in the absence of increased use of therapeutic injections.
Figures

Similar articles
-
An assessment of safe injection practices in health facilities in Swaziland.S Afr Med J. 2004 Mar;94(3):194-7. S Afr Med J. 2004. PMID: 15098279
-
Injection practices in Burkina Faso in 2000.Int J Qual Health Care. 2004 Aug;16(4):303-8. doi: 10.1093/intqhc/mzh052. Int J Qual Health Care. 2004. PMID: 15252004
-
Rapid assessment of injection practices in Cambodia, 2002.BMC Public Health. 2005 Jun 2;5:56. doi: 10.1186/1471-2458-5-56. BMC Public Health. 2005. PMID: 15929800 Free PMC article.
-
Could the WHO model list of essential medicines do more for the safe and appropriate use of injections?J Clin Pharmacol. 2004 Oct;44(10):1106-13. doi: 10.1177/0091270004268410. J Clin Pharmacol. 2004. PMID: 15342611 Review.
-
Sterilizable syringes: excessive risk or cost-effective option?Bull World Health Organ. 1999;77(10):812-9. Bull World Health Organ. 1999. PMID: 10593029 Free PMC article. Review.
Cited by
-
Safety in fragile, conflict-affected, and vulnerable settings: An evidence scanning approach for identifying patient safety interventions.J Glob Health. 2022 Feb 26;12:04018. doi: 10.7189/jogh.12.04018. eCollection 2022. J Glob Health. 2022. PMID: 35265329 Free PMC article.
-
The Impact of WHO Essential Medicines Policies on Inappropriate Use of Antibiotics.PLoS One. 2016 Mar 22;11(3):e0152020. doi: 10.1371/journal.pone.0152020. eCollection 2016. PLoS One. 2016. PMID: 27002977 Free PMC article.
-
Impact of harm minimization interventions on reducing blood-borne infection transmission and some injecting behaviors among people who inject drugs: an overview and evidence gap mapping.Addict Sci Clin Pract. 2024 Feb 4;19(1):9. doi: 10.1186/s13722-024-00439-9. Addict Sci Clin Pract. 2024. PMID: 38310293 Free PMC article.
-
Health and economic benefits of achieving hepatitis C virus elimination in Pakistan: A modelling study and economic analysis.PLoS Med. 2021 Oct 19;18(10):e1003818. doi: 10.1371/journal.pmed.1003818. eCollection 2021 Oct. PLoS Med. 2021. PMID: 34665815 Free PMC article.
References
-
- WHO-UNICEF-UNFPA Joint statement on the use of auto-disable syringes in immunization services. Geneva: World Health Organization, WHO document WHO/V&B/99.25; 1999.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

