Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status
- PMID: 16365053
- PMCID: PMC2718570
- DOI: 10.1093/jn/136.1.21
Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status
Abstract
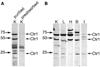
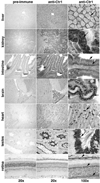
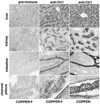
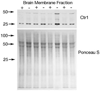
Studies were conducted to determine distribution of the copper transporter, Ctr1, a transmembrane protein responsible for cellular copper uptake, in adult mice and in suckling mice nursed by either copper-adequate (Cu+) or copper-deficient (Cu-) dams. Western immunoblot analyses, using immunopurified antibody, detected monomeric (23 kDa) and oligomeric forms of Ctr1 in the membrane fraction of several mouse organs. Immunohistochemical analyses detected abundant Ctr1 protein in liver canaliculi; kidney cortex tubules; small intestinal enterocytes; the choroid plexus and capillaries of brain; intercalated disks of heart; mature spermatozoa; epithelium of mammary ducts; and the pigment epithelium, outer limiting membrane, and outer plexiform layer of the retina. Duodenal Ctr1 distribution was different in the adult compared with suckling mice; adult mice demonstrated strong intracellular staining of the enterocyte, whereas apical staining predominated in suckling mice. In Cu- mice at postnatal d 16 (P16), Ctr1 staining was augmented in kidney, duodenum, and choroid plexus, compared with Cu+ mice. Brain immunoblot data indicated that Ctr1 protein in membrane fractions of Cu- mice was 56% higher than Cu+ mice. Cu- mice had lower hemoglobin (56% of Cu+), and lower copper concentration (% of Cu+) in liver (15%), brain (26%), and kidney (65%). These results suggest that Ctr1 protein is expressed in multiple tissues and found in higher levels in selected organs after perinatal copper deficiency. Enhanced Ctr1 levels and redistribution might compensate in part for the decrease in copper supply. Mechanisms for the enhancement in Ctr1 staining remain to be established.
Figures

References
-
- Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J Nutr. 2004;134:1003–1006. - PubMed
-
- Vulpe CD, Packman S. Cellular copper transport. Annu Rev Nutr. 1995;15:293–322. - PubMed
-
- Dancis A, Haile D, Yuan DS, Klausner RD. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem. 1994;269:25660–25667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

