Alpha4beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain
- PMID: 16365170
- PMCID: PMC2171310
- DOI: 10.1083/jcb.200503155
Alpha4beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain
Abstract
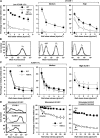
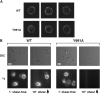
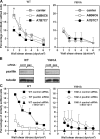
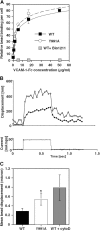
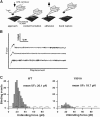
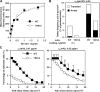
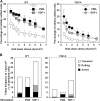
The capacity of integrins to mediate adhesiveness is modulated by their cytoplasmic associations. In this study, we describe a novel mechanism by which alpha4-integrin adhesiveness is regulated by the cytoskeletal adaptor paxillin. A mutation of the alpha4 tail that disrupts paxillin binding, alpha4(Y991A), reduced talin association to the alpha4beta1 heterodimer, impaired integrin anchorage to the cytoskeleton, and suppressed alpha4beta1-dependent capture and adhesion strengthening of Jurkat T cells to VCAM-1 under shear stress. The mutant retained intrinsic avidity to soluble or bead-immobilized VCAM-1, supported normal cell spreading at short-lived contacts, had normal alpha4-microvillar distribution, and responded to inside-out signals. This is the first demonstration that cytoskeletal anchorage of an integrin enhances the mechanical stability of its adhesive bonds under strain and, thereby, promotes its ability to mediate leukocyte adhesion under physiological shear stress conditions.
Figures



References
-
- Alon, R., and S. Feigelson. 2002. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin. Immunol. 14:93–104. - PubMed
-
- Ambroise, Y., B. Yaspan, M.H. Ginsberg, and D.L. Boger. 2002. Inhibitors of cell migration that inhibit intracellular paxillin/alpha4 binding: a well-documented use of positional scanning libraries. Chem. Biol. 9:1219–1226. - PubMed
-
- Benoit, M., D. Gabriel, G. Gerisch, and H.E. Gaub. 2000. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol. 2:313–317. - PubMed
-
- Berlin, C., E.L. Berg, M.J. Briskin, D.P. Andrew, P.J. Kilshaw, B. Holzmann, I.L. Weissman, A. Hamann, and E.C. Butcher. 1993. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 74:185–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

