The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice
- PMID: 16365293
- PMCID: PMC1323190
- DOI: 10.1073/pnas.0509438102
The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice
Abstract
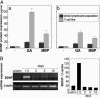
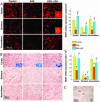
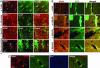
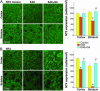
Neurotrophins (NTs) such as BDNF, NT-3, and NT-4 are important modulators of neuronal function and survival. Their expression in the CNS after various insults is thus of major therapeutic consequence. Glatiramer acetate [(GA) Copaxone], an approved drug for the treatment of multiple sclerosis, has been shown to induce Th2/3 cells that accumulate in the CNS, expressing in situ antiinflammatory cytokines and BDNF. In the present study, we investigated whether s.c. injections of GA, applied at various stages of experimental autoimmune encephalomyelitis, affect the expression of NTs, particularly BDNF, in the brain. In untreated experimental autoimmune encephalomyelitis mice, the expression of NTs was elevated shortly after disease appearance but subsequently declined below that of naive mice. In contrast, GA treatment led to sustained augmentation in the expression of BDNF, NT-3, and NT-4 in various brain regions as demonstrated by histological analysis of immunostained brain sections. GA treatment, even when started 45 days after disease induction, restored the impaired level of NTs to that of healthy mice. BDNF elevation after GA treatment was demonstrated on both protein and mRNA levels. Prominent staining was manifested not only by infiltrating GA-induced T cells, but also by CNS resident cells (neurons and astrocytes), indicative of a bystander therapeutic effect. Of importance, in GA-treated mice, intense BDNF expression was manifested by neuronal progenitors that migrated into lesions in injured regions. These results indicate that the immunomodulator GA exerts not only an antiinflammatory effect, but also enhances neuroprotection and regeneration of neural elements in the diseased brain.
Figures

References
-
- Behi, M. E., Dubucquoi, S., Lefranc, D., Zephir, H., De Seze, J., Vermersch, P. & Prin, L. (2005) Immunol. Lett. 96, 11-26. - PubMed
-
- Hellings, N., Raus, J. & Stinissen, P. (2002) Immunol. Res. 25, 27-51. - PubMed
-
- Bjartmar, C., Wujek, J. R. & Trapp, B. D. (2003) J. Neurol. Sci. 15, 165-171. - PubMed
-
- Lassmann, V., Gottmann, K. & Malcangio, M. (2003) Prog. Neurobiol. 69, 341-374. - PubMed
-
- Riley, C. P., Cope, T. C. & Buck, C. R. (2004) J. Mol. Histol. 35, 771-783. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

