Structure of the mid-region of tropomyosin: bending and binding sites for actin
- PMID: 16365313
- PMCID: PMC1323185
- DOI: 10.1073/pnas.0509269102
Structure of the mid-region of tropomyosin: bending and binding sites for actin
Abstract
Tropomyosin is a two-chain alpha-helical coiled coil whose periodic interactions with the F-actin helix are critical for thin filament stabilization and the regulation of muscle contraction. Here we deduce the mechanical and chemical basis of these interactions from the 2.3-A-resolution crystal structure of the middle three of tropomyosin's seven periods. Geometrically specific bends of the coiled coil, produced by clusters of core alanines, and variable bends about gaps in the core, produced by isolated alanines, occur along the molecule. The crystal packing is notable in signifying that the functionally important fifth period includes an especially favorable protein-binding site, comprising an unusual apolar patch on the surface together with surrounding charged residues. Based on these and other results, we have constructed a specific model of the thin filament, with the N-terminal halves of each period (i.e., the so-called "alpha zones") of tropomyosin axially aligned with subdomain 3 of each monomer in F-actin.
Figures
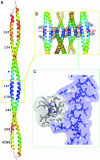



References
-
- Crick, F. H. C. (1953) Acta Crystallogr. 6, 689–697.
-
- Cohen, C. & Parry, D. A. D. (1990) Proteins Struct. Funct. Genet. 7, 1–15. - PubMed
-
- O'Shea, E. K., Klemm, J. D., Kim, P. S. & Alber, T. (1991) Science 254, 539–544. - PubMed
-
- McLachlan, A. D. & Stewart, M. (1976) J. Mol. Biol. 103, 271–298. - PubMed
-
- Phillips, G. N., Fillers, J. P. & Cohen, C. (1986) J. Mol. Biol. 192, 111–131. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

