Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series
- PMID: 16365317
- PMCID: PMC1323165
- DOI: 10.1073/pnas.0506627102
Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series
Abstract
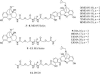
Given the mounting evidence for involvement of delta opioid receptors in the tolerance and physical dependence of mu opioid receptor agonists, we have investigated the possible physical interaction between mu and delta opioid receptors by using bivalent ligands. Based on reports of suppression of antinociceptive tolerance by the delta antagonist naltrindole (NTI), bivalent ligands [mu-delta agonist-antagonist (MDAN) series] that contain different length spacers, and pharmacophores derived from NTI and the mu agonist oxymorphone, have been synthesized and evaluated by intracerebroventricular (i.c.v.) administration in the tail-flick test in mice. In acute i.c.v. studies, the bivalent ligands functioned as agonists with potencies ranging from 1.6- to 45-fold greater than morphine. In contrast, the monovalent mu agonist analogues were substantially more potent than the MDAN congeners and were essentially equipotent with one another and oxymorphone. Pretreatment with NTI decreased the ED(50) values for MDAN-19 to a greater degree than for MDAN-16 but had no effect on MDAN-21. Chronic i.c.v. studies revealed that MDAN ligands whose spacer was 16 atoms or longer produced less dependence than either morphine or mu monovalent control MA-19. On the other hand, both physical dependence and tolerance were suppressed at MDAN spacer lengths of 19 atoms or greater. These data suggest that physical interaction between the mu and delta opioid receptors modulates mu-mediated tolerance and dependence. Because MDAN-21 was found to be 50-fold more potent than morphine by the i.v. route (i.v.), it offers a previously uncharacterized approach for the development of analgesics devoid of tolerance and dependence.
Figures
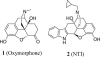
References
-
- Vaught, J. & Takemori, A. (1979) J. Pharmacol. Exp. Ther. 208, 86-94. - PubMed
-
- Vaught, J. L., Rothman, R. B. & Westfall, T. C. (1982) Life Sci. 30, 1443-1455. - PubMed
-
- Heyman, J. S. & Porreca, F. (1989) Prog. Opioid Res. 75, 467-470.
-
- Miyamoto, Y., Portoghese, P. S. & Takemori, A. E. (1993) J. Pharmacol. Exp. Ther. 264, 1141-1145. - PubMed
-
- Abdelhamid, E. E., Sultana, M., Portoghese, P. S. & Takemori, A. E. (1991) J. Pharmacol. Exp. Ther. 258, 299-303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

