Helminth-primed dendritic cells alter the host response to enteric bacterial infection
- PMID: 16365440
- PMCID: PMC4144328
- DOI: 10.4049/jimmunol.176.1.472
Helminth-primed dendritic cells alter the host response to enteric bacterial infection
Abstract
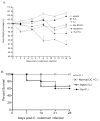
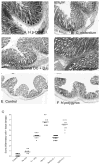
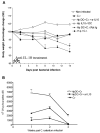
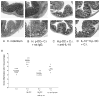
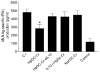
To examine whether intestinal helminth infection may be a risk factor for enteric bacterial infection, a murine model was established using the intestinal helminth Heligomosomoides polygyrus and a murine pathogen Citrobacter rodentium, which causes infectious colitis. Using this model we recently have shown that coinfection with the Th2-inducing H. polygyrus and C. rodentium promotes bacterial-associated disease and colitis. In this study, we expand our previous observations and examine the hypothesis that dendritic cells (DC) stimulated by helminth infection may play an important role in the regulation of the intestinal immune response to concurrent C. rodentium infection as well as in the modulation of the bacterial pathogenesis. We show that H. polygyrus infection induces DC activation and IL-10 expression, and that adoptive transfer of parasite-primed DC significantly impairs host protection to C. rodentium infection, resulting in an enhanced bacterial infection and in the development of a more severe colonic injury. Furthermore, we demonstrate that adoptive transfer of parasite-primed IL-10-deficient DCs fails to result in the development of a significantly enhanced C. rodentium-mediated colitis. Similarly, when the DC IL-10 response was neutralized by anti-IL-10 mAb treatment in mice that received parasite-primed DC, no deleterious effect of the parasite-primed DC on the host intestinal response to C. rodentium was detected. Thus, our results provide evidence to indicate that the H. polygyrus-dependent modulation of the host response to concurrent C. rodentium infection involves IL-10-producing DCs.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures




Similar articles
-
Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages.J Immunol. 2012 Aug 1;189(3):1459-66. doi: 10.4049/jimmunol.1200484. Epub 2012 Jun 25. J Immunol. 2012. PMID: 22732589 Free PMC article.
-
Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice.Infect Immun. 2005 Sep;73(9):5468-81. doi: 10.1128/IAI.73.9.5468-5481.2005. Infect Immun. 2005. PMID: 16113263 Free PMC article.
-
Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis.Mucosal Immunol. 2018 Jan;11(1):144-157. doi: 10.1038/mi.2017.20. Epub 2017 Mar 29. Mucosal Immunol. 2018. PMID: 28352104 Free PMC article.
-
Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses.J Immunol. 2012 Sep 1;189(5):2512-20. doi: 10.4049/jimmunol.1102892. Epub 2012 Jul 27. J Immunol. 2012. PMID: 22844110 Free PMC article.
-
Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis.J Immunol. 2007 Oct 1;179(7):4721-31. doi: 10.4049/jimmunol.179.7.4721. J Immunol. 2007. PMID: 17878371 Free PMC article.
Cited by
-
Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages.J Immunol. 2012 Aug 1;189(3):1459-66. doi: 10.4049/jimmunol.1200484. Epub 2012 Jun 25. J Immunol. 2012. PMID: 22732589 Free PMC article.
-
Modulation of dendritic cell responses by parasites: a common strategy to survive.J Biomed Biotechnol. 2010;2010:357106. doi: 10.1155/2010/357106. Epub 2010 Feb 24. J Biomed Biotechnol. 2010. PMID: 20204070 Free PMC article. Review.
-
Eosinophil-derived IL-10 supports chronic nematode infection.J Immunol. 2014 Oct 15;193(8):4178-87. doi: 10.4049/jimmunol.1400852. Epub 2014 Sep 10. J Immunol. 2014. PMID: 25210122 Free PMC article.
-
Helminths in alternative therapeutics of inflammatory bowel disease.Intest Res. 2025 Jan;23(1):8-22. doi: 10.5217/ir.2023.00059. Epub 2024 Jan 12. Intest Res. 2025. PMID: 39916482 Free PMC article. Review.
-
Chronic helminth infection promotes immune regulation in vivo through dominance of CD11cloCD103- dendritic cells.J Immunol. 2011 Jun 15;186(12):7098-109. doi: 10.4049/jimmunol.1003636. Epub 2011 May 16. J Immunol. 2011. PMID: 21576507 Free PMC article.
References
-
- Nokes C, Cooper ES, Robinson BA, Da B. Geohelminth infection and academic assessment in Jamaican children. Trans R Soc Trop Med Hyg. 1991;85:272–273. - PubMed
-
- Crompton DWT, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. - PubMed
-
- Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. - PubMed
-
- Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol. 2003;284:G385–G391. - PubMed
-
- Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

