Semi-rational design of (north)-methanocarba nucleosides as dual acting A(1) and A(3) adenosine receptor agonists: novel prototypes for cardioprotection
- PMID: 16366590
- PMCID: PMC2597460
- DOI: 10.1021/jm050726b
Semi-rational design of (north)-methanocarba nucleosides as dual acting A(1) and A(3) adenosine receptor agonists: novel prototypes for cardioprotection
Abstract
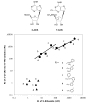
Ring-constrained adenosine analogues have been designed to act as dual agonists at tissue-protective A(1) and A(3) adenosine receptors (ARs). 9-Ribosides transformed into the ring-constrained (N)-methanocarba-2-chloro-5'-uronamides consistently lost affinity at A(1)/A(2A)ARs and gained at A(3)AR. Among 9-riboside derivatives, only N(6)-cyclopentyl and 7-norbornyl moieties were extrapolated for mixed A(1)/A(3) selectivity and rat/human A(3)AR equipotency. Consequently, 2 was balanced in affinity and potency at A(1)/A(3)ARs as envisioned and dramatically protected in an intact heart model of global ischemia and reperfusion.
Figures


References
-
- Yao L, Burbiel JC, Maass A, Müller CE. Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Expert Opin Emerging Drugs. 2003;8:537–576. - PubMed
-
- Liu GS, Richards SC, Olsson RA, Mullane K, Walsh RS, Downey JM. Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc Res. 1994;28:1057–1061. - PubMed
-
- Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, Knight DR. Novel N6-substituted adenosine 5′-N-methyluronamides with high selectivity for human adenosine A3 receptors reduce ischemic myocardial injury. AmJ Physiol Heart Circ Physiol. 2003;285:H2780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

