A new bisintercalating anthracycline with picomolar DNA binding affinity
- PMID: 16366602
- PMCID: PMC2522373
- DOI: 10.1021/jm050902g
A new bisintercalating anthracycline with picomolar DNA binding affinity
Abstract
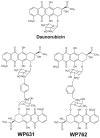
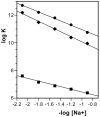
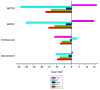
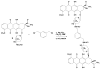
A new bisintercalating anthracycline (WP762) has been designed, in which monomeric units of daunorubicin have been linked through their amino groups on the daunosamine moieties using an m-xylenyl linker. Differential scanning calorimetry and UV melting experiments were used to measure the ultratight binding of WP762 to DNA. The binding constant for the interaction of WP762 with herring sperm DNA was determined to be 7.3 (+/-0.2) x 10(12) M(-1) at 20 degrees C. The large favorable binding free energy of -17.3 kcal mol(-1) was found to result from a large negative enthalpic contribution of -33.8 kcal mol(-1) and an opposing entropic term (-TDeltaS = +16.5 kcal mol(-1)). A comparative molecular modeling study rationalized the increased binding by the m-xylenyl linker of WP762 positioning in the DNA minor groove compared to the p-xylenyl linker found in WP631, the first bis-anthracycline of this type. The cytotoxicity of WP762 was compared to that of other anthracyclines in Jurkat T lymphocytes. These studies, together with an analysis of the cell-cycle traverse in the presence of WP762, suggest that in these cells the new drug is more cytotoxic than the structurally related WP631.
Figures






Similar articles
-
Structure-based design of a new bisintercalating anthracycline antibiotic.J Med Chem. 1997 Jan 31;40(3):261-6. doi: 10.1021/jm9607414. J Med Chem. 1997. PMID: 9022792
-
Ultratight DNA binding of a new bisintercalating anthracycline antibiotic.Biochemistry. 1998 Feb 17;37(7):1743-53. doi: 10.1021/bi9720742. Biochemistry. 1998. PMID: 9485299
-
Exploiting anthracycline scaffold for designing DNA-targeting agents.Methods Enzymol. 2001;340:529-55. doi: 10.1016/s0076-6879(01)40441-1. Methods Enzymol. 2001. PMID: 11494869 No abstract available.
-
Analysis of the effects of daunorubicin and WP631 on transcription.Curr Med Chem. 2001 Jan;8(1):1-8. doi: 10.2174/0929867013373976. Curr Med Chem. 2001. PMID: 11172687 Review.
-
Drug--DNA interactions.Curr Opin Struct Biol. 1998 Jun;8(3):314-20. doi: 10.1016/s0959-440x(98)80064-x. Curr Opin Struct Biol. 1998. PMID: 9666327 Review.
Cited by
-
Evaluation of molecular descriptors for antitumor drugs with respect to noncovalent binding to DNA and antiproliferative activity.BMC Pharmacol. 2009 Sep 16;9:11. doi: 10.1186/1471-2210-9-11. BMC Pharmacol. 2009. PMID: 19758437 Free PMC article.
-
POT1 stability and binding measured by fluorescence thermal shift assays.PLoS One. 2021 Mar 30;16(3):e0245675. doi: 10.1371/journal.pone.0245675. eCollection 2021. PLoS One. 2021. PMID: 33784306 Free PMC article.
-
Entropically-driven binding of mithramycin in the minor groove of C/G-rich DNA sequences.Nucleic Acids Res. 2007;35(7):2215-26. doi: 10.1093/nar/gkm037. Epub 2007 Mar 16. Nucleic Acids Res. 2007. PMID: 17369273 Free PMC article.
-
Study on the interaction between the 1,4,5,8-naphthalene diimide-spermine conjugate (NDIS) and DNA using a spectroscopic approach and molecular docking.Medchemcomm. 2017 Sep 26;8(11):2079-2092. doi: 10.1039/c7md00389g. eCollection 2017 Nov 1. Medchemcomm. 2017. PMID: 30108725 Free PMC article.
-
Tuning the DNA conformational perturbations induced by cytotoxic platinum-acridine bisintercalators: effect of metal cis/trans isomerism and DNA threading groups.J Med Chem. 2008 Jun 12;51(11):3069-72. doi: 10.1021/jm8003569. Epub 2008 May 6. J Med Chem. 2008. PMID: 18457380 Free PMC article.
References
-
- Booser DJ, Hortobagyi GN. Anthracycline antibiotics in cancer therapy. Focus on drug resistance. Drugs. 1994;47:223–258. - PubMed
-
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews. 2004;56:185–229. - PubMed
-
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology. 1999;57:727–741. - PubMed
-
- Binaschi M, Bigioni M, Cipollone A, Rossi C, Goso C, Maggi CA, Capranico G, Animati F. Anthracyclines: selected new developments. Current Medicinal Chemistry - Anti-Cancer Agents. 2001;1:113–130. - PubMed
-
- Chaires JB, Leng F, Przewloka T, Fokt I, Ling YH, Perez-Soler R, Priebe W. Structure-based design of a new bisintercalating anthracycline antibiotic. Journal of Medicinal Chemistry. 1997;40:261–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

