Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes
- PMID: 16367870
- PMCID: PMC1435360
- DOI: 10.1111/j.1462-5822.2005.00604.x
Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes
Abstract
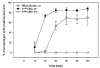
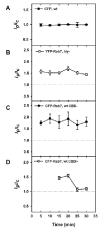
The bacterial pathogen Listeria monocytogenes (Lm) evades the antimicrobial mechanisms of macrophages by escaping from vacuoles to the cytosol, through the action of the cytolysin listeriolysin O (LLO). Because of heterogeneities in the timing and efficiency of escape, important questions about the contributions of LLO to Lm vacuole identity and trafficking have been inaccessible. Expression of cyan fluorescent protein (CFP)-labelled endocytic membrane markers in macrophages along with a yellow fluorescent protein (YFP)-labelled indicator of Lm entry to the cytosol identified compartments lysed by bacteria. Lm escaped from Rab5a-negative, lysosome-associated membrane protein-1 (LAMP1)-negative, Rab7-positive, phosphatidylinositol 3-phosphate [PI(3)P]-positive vacuoles. Lm vacuoles did not label with YFP-Rab5a unless the bacteria were first opsonized with IgG. Wild-type Lm delayed vacuole fusion with LAMP1-positive lysosomes, relative to LLO-deficient Lm. Bacteria prevented from expressing LLO until their arrival into LAMP1-positive lysosomes escaped inefficiently. Thus, the LLO-dependent delay of Lm vacuole fusion with lysosomes affords Lm a competitive edge against macrophage defences by providing bacteria more time in organelles they can penetrate.
Figures







References
-
- Alvarez-Dominguez C, Stahl PD. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J Biol Chem. 1999;274:11459–11462. - PubMed
-
- Alvarez-Dominguez C, Roberts R, Stahl PD. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J Cell Sci. 1997;110:731–743. - PubMed
-
- Conte MP, Petrone G, Longhi C, Valenti P, Morelli R, Superti F, Seganti L. The effects of inhibitors of vacuolar acidification on the release of Listeria monocytogenes from phagosomes of Caco-2 cells. J Med Microbiol. 1996;44:418–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

