Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis
- PMID: 16367902
- PMCID: PMC11159386
- DOI: 10.1111/j.1349-7006.2005.00130.x
Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis
Abstract
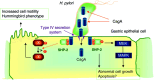
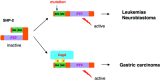
Infection with CagA-positive Helicobacter pylori is associated with the development of gastric adenocarcinoma. The CagA gene product CagA is injected directly from the bacterium into the bacterium-attached gastric epithelial cells via the type-IV secretion system. Upon membrane localization and subsequent tyrosine phosphorylation by Src family kinases, CagA functions as a scaffolding adaptor and interacts with a number of host proteins that regulate cell growth, cell motility and cell polarity in both CagA phosphorylation-dependent and phosphorylation-independent manners. Of special interest is the interaction of CagA with the SHP-2 tyrosine phosphatase, gain-of-function mutations that of which have recently been found in a variety of human malignancies. The CagA-SHP-2 interaction is entirely dependent on CagA tyrosine phosphorylation and, through the complex formation, SHP-2 is catalytically activated and induces morphological transformation with elevated cell motility. Intriguingly, structural diversity of the tyrosine phosphorylation sites of CagA accounts for the differential activity of individual CagA to bind and activate SHP-2. Deregulation of SHP-2 and other intracellular signaling molecules by H. pylori CagA may predispose cells to accumulate multiple genetic and epigenetic changes involved in gastric carcinogenesis. Furthermore, the differential potential of individual CagA to disturb cellular functions indicates that H. pylori strains carrying biologically more active CagA are more virulent than those with less active CagA and are more closely associated with gastric carcinoma.
(Cancer Sci 2005; 96: 835 - 843).
Figures



Similar articles
-
Helicobacter pylori CagA -- a bacterial intruder conspiring gastric carcinogenesis.Int J Cancer. 2006 Sep 15;119(6):1217-23. doi: 10.1002/ijc.21831. Int J Cancer. 2006. PMID: 16557568 Review.
-
The role of Helicobacter pylori CagA in gastric carcinogenesis.Int J Hematol. 2006 Nov;84(4):301-8. doi: 10.1532/IJH97.06166. Int J Hematol. 2006. PMID: 17118755 Review.
-
Deregulation of SHP-2 tyrosine phosphatase by the Helicobacter pylori virulence factor CagA.Keio J Med. 2002 Dec;51 Suppl 2:26-32. doi: 10.2302/kjm.51.supplement2_26. Keio J Med. 2002. PMID: 12528933
-
Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites.Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14428-33. doi: 10.1073/pnas.222375399. Epub 2002 Oct 21. Proc Natl Acad Sci U S A. 2002. PMID: 12391297 Free PMC article.
-
Systematic analysis of phosphotyrosine antibodies recognizing single phosphorylated EPIYA-motifs in CagA of East Asian-type Helicobacter pylori strains.BMC Microbiol. 2016 Sep 2;16(1):201. doi: 10.1186/s12866-016-0820-6. BMC Microbiol. 2016. PMID: 27590005 Free PMC article.
Cited by
-
Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation.Cancer Sci. 2009 Oct;100(10):1786-93. doi: 10.1111/j.1349-7006.2009.01257.x. Epub 2009 Jun 23. Cancer Sci. 2009. PMID: 19622105 Free PMC article. Review.
-
Helicobacter pylori-related risk predictors of gastric cancer: The latest models, challenges, and future prospects.Cancer Med. 2020 Jul;9(13):4808-4822. doi: 10.1002/cam4.3068. Epub 2020 May 4. Cancer Med. 2020. PMID: 32363738 Free PMC article. Review.
-
Integrin but not CEACAM receptors are dispensable for Helicobacter pylori CagA translocation.PLoS Pathog. 2018 Oct 26;14(10):e1007359. doi: 10.1371/journal.ppat.1007359. eCollection 2018 Oct. PLoS Pathog. 2018. PMID: 30365569 Free PMC article.
-
Helicobacter Pylori associated global gastric cancer burden.Front Biosci (Landmark Ed). 2009 Jan 1;14(4):1490-504. doi: 10.2741/3320. Front Biosci (Landmark Ed). 2009. PMID: 19273142 Free PMC article. Review.
-
Bacterial oncogenesis in the colon.Future Microbiol. 2013 Apr;8(4):445-60. doi: 10.2217/fmb.13.17. Future Microbiol. 2013. PMID: 23534358 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

