Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2
- PMID: 16367905
- PMCID: PMC11159771
- DOI: 10.1111/j.1349-7006.2005.00126.x
Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2
Abstract
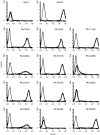
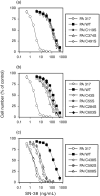
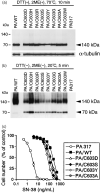
Breast cancer resistance protein (BCRP/ABCG2) is a half-molecule ATP-binding cassette transporter that we have previously suggested might function as a homodimer, bridged by disulfide bonds. In the present study, we carried out cysteine-scanning mutagenesis, substituting Ser for Cys, and established 12 PA317 transfectants expressing BCRP mutants with possible disruptions to their S-S bonds. Western blot analysis of BCRP from the wild-type transfectants (PA/WT) confirmed that the wild-type protein migrates as a 140-kDa dimer under non-reducing conditions, but as a 70-kDa monomer under reducing conditions. However, under non-reducing conditions the BCRP-C603S mutant migrated both as a 70-kDa monomer and a 140-kDa dimer, whereas all other mutant BCRP migrated only as dimers. PA317 cells transfected with C603S-BCRP (PA/C603S) showed either similar or only marginally lower SN-38 resistance than PA/WT cells, despite the reduced levels of BCRP dimer in these cells. Moreover, the degree of SN-38 resistance in the mutant BCRP transfectants was found to be associated with the monomer expression levels under reducing conditions. Reverse transcription-polymerase chain reaction analysis showed that the BCRP mRNA levels were similar in the transfectants. We subsequently generated six C603X mutants of BCRP (X=D, H, R, Y, A and W) and carried out western blot analysis and drug sensitivity assays. The results were equivalent to those from the PA/C603S cells, with some variations that again corresponded to the monomer levels. Our findings suggest that Cys-603 is an important residue in the covalent bridge between BCRP monomers but that a functioning unit of BCRP may not necessarily require covalent linkages.
(Cancer Sci 2005; 96: 866-872).
Figures


Similar articles
-
BCRP/ABCG2 confers anticancer drug resistance without covalent dimerization.Cancer Sci. 2010 Aug;101(8):1813-21. doi: 10.1111/j.1349-7006.2010.01605.x. Epub 2010 Apr 28. Cancer Sci. 2010. PMID: 20518788 Free PMC article.
-
Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization.Int J Cancer. 2002 Feb 10;97(5):626-30. doi: 10.1002/ijc.10100. Int J Cancer. 2002. PMID: 11807788
-
Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants.Int J Cancer. 2003 Dec 10;107(5):757-63. doi: 10.1002/ijc.11484. Int J Cancer. 2003. PMID: 14566825
-
Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2).Oncogene. 2003 Oct 20;22(47):7340-58. doi: 10.1038/sj.onc.1206938. Oncogene. 2003. PMID: 14576842 Review.
-
Functional SNPs of the breast cancer resistance protein-therapeutic effects and inhibitor development.Cancer Lett. 2006 Mar 8;234(1):73-80. doi: 10.1016/j.canlet.2005.04.039. Epub 2005 Nov 21. Cancer Lett. 2006. PMID: 16303243 Review.
Cited by
-
Cancer drug resistance: redox resetting renders a way.Oncotarget. 2016 Jul 5;7(27):42740-42761. doi: 10.18632/oncotarget.8600. Oncotarget. 2016. PMID: 27057637 Free PMC article. Review.
-
Recombinant synthesis of human ABCG2 expressed in the yeast Saccharomyces cerevisiae: an experimental methodological study.Protein J. 2011 Mar;30(3):201-11. doi: 10.1007/s10930-011-9321-5. Protein J. 2011. PMID: 21424391
-
Human bile acid transporter ASBT (SLC10A2) forms functional non-covalent homodimers and higher order oligomers.Biochim Biophys Acta Biomembr. 2018 Mar;1860(3):645-653. doi: 10.1016/j.bbamem.2017.11.016. Epub 2017 Dec 1. Biochim Biophys Acta Biomembr. 2018. PMID: 29198943 Free PMC article.
-
The structure of the human ABC transporter ABCG2 reveals a novel mechanism for drug extrusion.Sci Rep. 2017 Oct 23;7(1):13767. doi: 10.1038/s41598-017-11794-w. Sci Rep. 2017. PMID: 29061978 Free PMC article.
-
ABCG2 Transporter: From Structure to Function-Current Insights and Open Questions.Int J Mol Sci. 2025 Jun 25;26(13):6119. doi: 10.3390/ijms26136119. Int J Mol Sci. 2025. PMID: 40649897 Free PMC article. Review.
References
-
- Chen C‐J, Chin JE, Ueda K et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P‐glycoprotein) gene from multidrug‐resistant human cells. Cell 1986; 47: 381–9. - PubMed
-
- Ueda K, Clark DP, Chen C‐J, Roninson IB, Gottesman MM, Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem 1987; 262: 505–8. - PubMed
-
- Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Annu Rev Genet 1995; 29: 607–49. - PubMed
-
- Cole SPC, Bhardwaj G, Gerlach JH et al. Overexpression of a transporter gene in a multidrug‐resistant human lung cancer cell line. Science 1992; 258: 1650–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

