Inhibitory effect of dietary monoglucosylceramide 1-O-beta-glucosyl-N-2'-hydroxyarachidoyl-4,8-sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in F344 rats
- PMID: 16367907
- PMCID: PMC11158130
- DOI: 10.1111/j.1349-7006.2005.00127.x
Inhibitory effect of dietary monoglucosylceramide 1-O-beta-glucosyl-N-2'-hydroxyarachidoyl-4,8-sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in F344 rats
Abstract
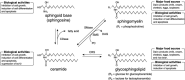
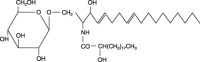
Sphingolipids display a wide spectrum of biological activities, including cell growth, differentiation and apoptosis. However, precise mechanisms by which these compounds exert anticancer or cancer-preventive effects are not known. In the present study, we evaluated the preventive efficacy of enriched dietary monoglucosylceramide 1-O-beta-glucosyl-N-2'-hydroxyarachidoyl-4,8-sphingadienine (G(1)CM) on 1,2-dimethylhydrazine (DMH)-induced aberrant crypt foci (ACF) and beta-catenin-accumulated crypt (BCAC) formation in F344 rats during initiation stage. We also examined whether G(1)CM affects cell proliferation and apoptosis in these lesions. Pure G(1)CM was isolated from rice bran. Forty-two rats were divided randomly into five experimental groups. Rats in groups 1-3 were given subcutaneous injections of DMH (40 mg/kg body weight) once a week for 2 weeks. One week before the first injection of DMH, rats in groups 2 and 3 were fed a diet containing 200 and 1,000 p.p.m. G(1)CM, respectively, for 5 weeks. Rats in group 4 were fed a diet containing 1,000 p.p.m. G(1)CM. Rats in group 5 were given the basal diet alone and served as untreated controls. The experiment was terminated 5 weeks after the start. Dietary G(1)CM at both doses (groups 2 and 3) significantly inhibited the induction of ACF and BCAC (P<0.001) when compared to group 1 treated with DMH alone. In groups 2 and 3, the proliferating cell nuclear antigen labeling indices of epithelial cells in ACF and BCAC were also lower than in group 1 (P<0.0001 for ACF, P<0.05 for BCAC). These results, that dietary G(1)CM has possible chemopreventive effects in the present short-term colon carcinogenesis bioassays, suggest that longer exposure may cause suppression of tumor development.
(Cancer Sci 2005; 96: 876-881).
Figures

References
-
- Vesper H, Schmelz EM, Nikolova‐Karakashian MN, Dillehay DL, Lynch DV, Merrill AH Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr 1999; 129: 1239–50. - PubMed
-
- Merrill AH Jr, Schmelz EM, Dillehay DL et al. Sphingolipids − the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol 1997; 142: 208–25. - PubMed
-
- Spiegel S, Merrill AH Jr. Sphingolipid metabolism and cell growth regulation. FASEB J 1996; 10: 1388–97. - PubMed
-
- Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr 1986; 116: 50–8. - PubMed
-
- Fujino Y, Ohnishi M, Ito S. Molecular species of ceramide and mono‐, di‐, tri‐, and tetraglycosylceramide in bran and endosperm of rice grains. Agric Biol Chem 1985; 49: 2753–62.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

