Inhibition of classical complement activation attenuates liver ischaemia and reperfusion injury in a rat model
- PMID: 16367929
- PMCID: PMC1809558
- DOI: 10.1111/j.1365-2249.2005.02958.x
Inhibition of classical complement activation attenuates liver ischaemia and reperfusion injury in a rat model
Abstract
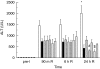
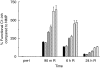
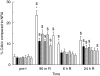
Activation of the complement system contributes to the pathogenesis of ischaemia/reperfusion (I/R) injury. We evaluated inhibition of the classical pathway of complement using C1-inhibitor (C1-inh) in a model of 70% partial liver I/R injury in male Wistar rats (n = 35). C1-inh was administered at 100, 200 or 400 IU/kg bodyweight, 5 min before 60 min ischaemia (pre-I) or 5 min before 24 h reperfusion (end-I). One hundred IU/kg bodyweight significantly reduced the increase of plasma levels of activated C4 as compared to albumin-treated control rats and attenuated the increase of alanine aminotransferase (ALT). These effects were not better with higher doses of C1-inh. Administration of C1-inh pre-I resulted in lower ALT levels and higher bile secretion after 24 h of reperfusion than administration at end-I. Immunohistochemical assessment indicated that activated C3, the membrane attack complex C5b9 and C-reactive protein (CRP) colocalized in hepatocytes within midzonal areas, suggesting CRP is a mediator of I/R-induced, classical complement activation in rats. Pre-ischaemic administration of C1-inh is an effective pharmacological intervention to protect against liver I/R injury.
Figures



References
-
- Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;49:146–51. - PubMed
-
- Yasuda M, Takeuchi K, Hiruma M, et al. The complement system in ischemic heart disease. Circulation. 1990;81:156. - PubMed
-
- Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FDJ. Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–8. - PubMed
-
- Hebert LA, Cosio FG, Birmingham DJ. The role of the complement system in renal injury. Seminars Nephrol. 1992;12:408–27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

