Activation of c-Jun N-terminal kinase (JNK) signalling in experimentally induced gastric lesions in rats
- PMID: 16367930
- PMCID: PMC1809559
- DOI: 10.1111/j.1365-2249.2005.02959.x
Activation of c-Jun N-terminal kinase (JNK) signalling in experimentally induced gastric lesions in rats
Abstract
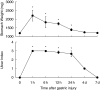
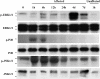
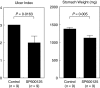
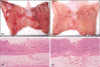
The c-Jun N-terminal kinase (JNK) participates in intracellular signalling cascades that mediate inflammatory responses. Therefore, the JNK signalling may be involved in gastric injury and inhibition of this pathway may form the basis of a new strategy for the treatment of gastric injury. The aim of this study was to determine whether JNK participates in the formation of gastric lesions in an experimental model. Acute gastric injury was induced in Sprague-Dawley rats by intragastric administration of 100% ethanol. The amount of phospho-JNK in the rat stomach was determined using immunohistochemistry and Western analysis. Animals received subcutaneous injections of a specific JNK inhibitor SP600125 or vehicle and the extent of mucosal damage in the stomach was determined. Western analysis revealed early phosphorylation of JNK and, to a lesser extent, p38 as well as late phosphorylation of the p42/44 extracellular signal-related kinases during the development of gastric lesions. JNK was phosphorylated in epithelial cells and in occasional mononuclear cells present at lesion sites. These cells were rarely found in samples from control specimens. Treatment with SP600125 significantly reduced the extent of gastric lesions. These findings indicate that experimental gastric injury is associated with activation of the JNK signalling pathway, and also suggest that JNK inhibitors may play a role in the treatment of gastric injury in humans.
Figures


Similar articles
-
Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory bowel disease.Int J Mol Med. 2006 Mar;17(3):449-55. Int J Mol Med. 2006. PMID: 16465391
-
Expression of apoptosis-associated microRNAs in ethanol-induced acute gastric mucosal injury via JNK pathway.Alcohol. 2013 Sep;47(6):481-93. doi: 10.1016/j.alcohol.2013.05.005. Epub 2013 Jul 3. Alcohol. 2013. PMID: 23830200
-
Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes.Eur J Pharmacol. 2005 Jan 31;508(1-3):31-45. doi: 10.1016/j.ejphar.2004.12.006. Epub 2005 Jan 13. Eur J Pharmacol. 2005. PMID: 15680252
-
IKappaB-kinase/nuclear factor-kappaB signaling prevents thermal injury-induced gut damage by inhibiting c-Jun NH2-terminal kinase activation.Crit Care Med. 2007 May;35(5):1332-40. doi: 10.1097/01.CCM.0000261891.30360.F0. Crit Care Med. 2007. PMID: 17414734
-
Inhibitors of c-Jun N-terminal kinases: JuNK no more?Biochim Biophys Acta. 2008 Jan;1784(1):76-93. doi: 10.1016/j.bbapap.2007.09.013. Epub 2007 Oct 11. Biochim Biophys Acta. 2008. PMID: 17964301 Free PMC article. Review.
Cited by
-
JNK pathway activation is controlled by Tao/TAOK3 to modulate ethanol sensitivity.PLoS One. 2012;7(12):e50594. doi: 10.1371/journal.pone.0050594. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227189 Free PMC article.
-
Extracellular signal-regulated kinase 1- and 2-mediated gastric mucosal injury and repair in gastric ischemia-reperfusion of rats.J Gastroenterol. 2006 Dec;41(12):1158-68. doi: 10.1007/s00535-006-1902-2. Epub 2007 Feb 6. J Gastroenterol. 2006. PMID: 17287895
-
Focused examination of the intestinal lamina propria yields greater molecular insight into mechanisms underlying SIV induced immune dysfunction.PLoS One. 2012;7(4):e34561. doi: 10.1371/journal.pone.0034561. Epub 2012 Apr 12. PLoS One. 2012. PMID: 22511950 Free PMC article.
-
Rutaecarpine Ameliorates Ethanol-Induced Gastric Mucosal Injury in Mice by Modulating Genes Related to Inflammation, Oxidative Stress and Apoptosis.Front Pharmacol. 2020 Nov 26;11:600295. doi: 10.3389/fphar.2020.600295. eCollection 2020. Front Pharmacol. 2020. PMID: 33324227 Free PMC article.
References
-
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–5. - PubMed
-
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. - PubMed
-
- Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. - PubMed
-
- Kallunki T, Su B, Tsigelny I, Sluss H, Derijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. - PubMed
-
- Jain J, Valge-Archer VE, Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992;148:1240–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

