T cell receptor-transgenic primary T cells as a tool for discovery of leukaemia-associated antigens
- PMID: 16367937
- PMCID: PMC1809573
- DOI: 10.1111/j.1365-2249.2005.02967.x
T cell receptor-transgenic primary T cells as a tool for discovery of leukaemia-associated antigens
Abstract
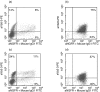
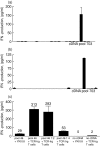
Identification of a broad array of leukaemia-associated antigens is a crucial step towards immunotherapy of haematological malignancies. However, it is frequently hampered by the decrease of proliferative potential and functional activity of T cell clones used for screening procedures. Transfer of the genes encoding the T cell receptor (TCR) alpha and beta chains of leukaemia-specific clones into primary T cells may help to circumvent this obstacle. In this study, transfer of two minor histocompatibility antigen (minor H antigen)-specific TCRs was performed and the feasibility of the use of TCR-transgenic T cells for identification of minor H antigens through cDNA library screening was investigated. We found that TCR-transgenic cells acquired the specificity of the original clones and matched their sensitivity. Moreover, the higher scale of cytokine-production by TCR-transgenic T cells permits the detection of either small amounts of antigen-positive cells or cells expressing low amounts of an antigen. When applied in equal numbers, TCR-transgenic T cells and the original T cell clones produced similar results in the screening of a cDNA library. However, the use of increased numbers of TCR-transgenic T cells allowed detection of minute amounts of antigen, barely discernible by the T cell clone. In conclusion, TCR-transfer generates a large amount of functional antigen-specific cells suitable for screening of cDNA expression libraries for identification of cognate antigens.
Figures


References
-
- Champlin R, Gale RP. Bone marrow transplantation: its biology and role as treatment for acute and chronic leukemias. Ann NY Acad Sci. 1987;511:447–58. - PubMed
-
- Antin JH. Graft-versus-leukemia: no longer an epiphenomenon. Blood. 1993;82:2273–7. - PubMed
-
- Spierings E, Wieles B, Goulmy E. Minor histocompatibility antigens − big in tumour therapy. Trends Immunol. 2004;25:56–60. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

