Constitutive activity of a UV cone opsin
- PMID: 16368093
- PMCID: PMC1661692
- DOI: 10.1016/j.febslet.2005.12.002
Constitutive activity of a UV cone opsin
Abstract
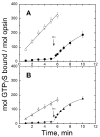
Vertebrate visual pigment proteins contain a conserved carboxylic acid residue in the third transmembrane helix. In rhodopsin, Glu113 serves as a counterion to the positively charged protonated Schiff base formed by 11-cis retinal attached to Lys296. Activation involves breaking of this ion pair. In UV cone pigments, the retinyl Schiff base is unprotonated, and hence such a salt bridge is not present; yet the pigment is inactive in the dark. Mutation of Glu108, which corresponds to rhodopsin's Glu113, to Gln yields a pigment that remains inactive in the dark. The apoproteins of both the wild-type and mutant, however, are constitutively active with the mutant being of significantly higher activity. Thus, one important role for preserving the negatively charged glutamate in the third helix of UV pigments is to maintain a less active opsin in a manner similar to rhodopsin. Ligand binding itself in the absence of a salt bridge is sufficient for deactivation.
Figures


Similar articles
-
Regulation of phototransduction in short-wavelength cone visual pigments via the retinylidene Schiff base counterion.Biochemistry. 2001 Nov 20;40(46):13760-6. doi: 10.1021/bi015584b. Biochemistry. 2001. PMID: 11705364
-
Photochemistry of the primary event in short-wavelength visual opsins at low temperature.Biochemistry. 1999 Aug 31;38(35):11287-97. doi: 10.1021/bi990968b. Biochemistry. 1999. PMID: 10471278
-
Regulation of photoactivation in vertebrate short wavelength visual pigments: protonation of the retinylidene Schiff base and a counterion switch.Biochemistry. 2007 May 8;46(18):5330-40. doi: 10.1021/bi700138g. Epub 2007 Apr 18. Biochemistry. 2007. PMID: 17439245
-
Cone visual pigments.Biochim Biophys Acta. 2014 May;1837(5):664-73. doi: 10.1016/j.bbabio.2013.08.009. Epub 2013 Sep 7. Biochim Biophys Acta. 2014. PMID: 24021171 Review.
-
Divergent mechanisms for the tuning of shortwave sensitive visual pigments in vertebrates.Photochem Photobiol Sci. 2004 Aug;3(8):713-20. doi: 10.1039/b314693f. Epub 2004 Mar 22. Photochem Photobiol Sci. 2004. PMID: 15295625 Review.
Cited by
-
Assays for inverse agonists in the visual system.Methods Enzymol. 2010;485:213-24. doi: 10.1016/B978-0-12-381296-4.00012-9. Methods Enzymol. 2010. PMID: 21050919 Free PMC article.
-
Light prevents exogenous 11-cis retinal from maintaining cone photoreceptors in chromophore-deficient mice.Invest Ophthalmol Vis Sci. 2011 Apr 14;52(5):2412-6. doi: 10.1167/iovs.10-6437. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21228385 Free PMC article.
-
In vitro assays of rod and cone opsin activity: retinoid analogs as agonists and inverse agonists.Methods Mol Biol. 2010;652:85-94. doi: 10.1007/978-1-60327-325-1_4. Methods Mol Biol. 2010. PMID: 20552423 Free PMC article.
-
Retinal Attachment Instability Is Diversified among Mammalian Melanopsins.J Biol Chem. 2015 Nov 6;290(45):27176-27187. doi: 10.1074/jbc.M115.666305. Epub 2015 Sep 28. J Biol Chem. 2015. PMID: 26416885 Free PMC article.
-
Modulation of molecular interactions and function by rhodopsin palmitylation.Biochemistry. 2009 May 26;48(20):4294-304. doi: 10.1021/bi900417b. Biochemistry. 2009. PMID: 19348429 Free PMC article.
References
-
- Kochendoerfer GG, Lin SW, Sakmar TP, Mathies RA. How color visual pigments are tuned. Trends Biochem Sci. 1999;24:300–305. - PubMed
-
- Starace DM, Knox BE. Cloning and expression of a Xenopus short wavelength cone pigment. Exp Eye Res. 1998;67:209–220. - PubMed
-
- Zhukovsky EA, Oprian DD. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989;246:928–930. - PubMed
-
- Honig B, Ebrey TG. Protein-chromophore interactions as spectroscopic and photochemical determinants. Methods Enzymol. 1982;88:462–470.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

