The mannose-binding lectin: a prototypic pattern recognition molecule
- PMID: 16368230
- PMCID: PMC7126801
- DOI: 10.1016/j.coi.2005.11.014
The mannose-binding lectin: a prototypic pattern recognition molecule
Abstract
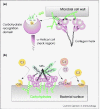
The innate immune system is comprised of a sophisticated network of recognition and effector molecules that act together to protect the host in the first minutes or hours of exposure to an infectious challenge. The mannose-binding lectin (MBL) is an evolutionary conserved circulating host defense protein that acts as a broad-spectrum recognition molecule against a wide variety of infectious agents. Target binding triggers the MBL pathway of complement activation. MBL can be considered conceptually as an 'ante-antibody' because it has a role in mammals during the lag period that is required to develop an antibody response against infectious agents. Additionally, there are MBL-like homologues in animals that lack adaptive immunity that activate a primitive complement system, and under these circumstances these MBL-like molecules play an analogous role to antibodies in higher animals. These molecules might be considered to be functional antecedents of antibodies. Recent work also indicates that MBL recognizes altered self-antigens, and as such MBL has a role that extends beyond a traditional role in first line host defense as it appears to play a role as a modulator of inflammation.
Figures
References
-
- Hoffmann J.A., Kafatos F.C., Janeway C.A., Ezekowitz R.A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. - PubMed
-
- Hansen T.K. Mannose-binding lectin (MBL) and vascular complications in diabetes. Horm Metab Res. 2005;37:95–102. - PubMed
-
- Madsen H.O., Satz M.L., Hogh B., Svejgaard A., Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161:3169–3175. - PubMed
-
- Uemura K., Saka M., Nakagawa T., Kawasaki N., Thiel S., Jensenius J.C., Kawasaki T. L-MBP is expressed in epithelial cells of mouse small intestine. J Immunol. 2002;169:6945–6950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

