Identification of a new spore wall protein from Encephalitozoon cuniculi
- PMID: 16368977
- PMCID: PMC1346661
- DOI: 10.1128/IAI.74.1.239-247.2006
Identification of a new spore wall protein from Encephalitozoon cuniculi
Abstract
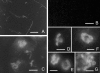
Microsporidia form environmentally resistant spores that are critical for their host-to-host transmission and persistence in the environment. The spore walls of these organisms are composed of two layers, the exospore and the endospore. Two spore wall proteins (SWP1 and SWP2) have been previously identified in members of the Encephalitozoonidae family. These proteins localize to the exospore. The endospore is known to contain chitin, and a putative glycosylphosphatidylinositol (GPI)-anchored chitin deacetylase has been localized to the plasmalemma-endospore interface. Using proteomic techniques, we have identified a new spore wall protein (SWP3) that is located in the endospore. The gene for this protein is located on chromosome 1 and corresponds to the open reading frame ECU01_1270. SWP3 is predicted to have a signal peptide and to be GPI anchored. Consistent with these modifications, two-dimensional electrophoresis demonstrated that SWP3 has an acidic pI and a molecular mass of <20 kDa. By immunoelectron microscopy, this protein was found on the cell surface during sporogony and in the endospore in mature spores. SWP3 has several potential O-glycosylation sites, and it is possible that it is a mannosylated protein like the major polar tube protein (PTP1).
Figures




References
-
- Biderre, C., A. Mathis, P. Deplazes, R. Weber, G. Metenier, and C. P. Vivares. 1999. Molecular karyotype diversity in the microsporidian Encephalitozoon cuniculi. Parasitology 118:439-445. - PubMed
-
- Bigliardi, E., M. G. Selmi, P. Lupetti, S. Corona, S. Gatti, M. Scaglia, and L. Sacchi. 1996. Microsporidian spore wall: ultrastructural findings on Encephalitozoon hellem exospore. J. Eukaryot. Microbiol. 43:181-186. - PubMed
-
- Brosson, D., L. Kuhn, G. Prensier, C. P. Vivares, and C. Texier. 2005. The putative chitin deacetylase of Encephalitozoon cuniculi: a surface protein implicated in microsporidian spore-wall formation. FEMS Microbiol. Lett. 247:81-90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

