Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans
- PMID: 16368999
- PMCID: PMC1346595
- DOI: 10.1128/IAI.74.1.435-441.2006
Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans
Abstract
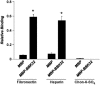
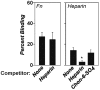
Borrelia burgdorferi, the agent of Lyme disease, causes a multisystemic illness that can affect the skin, heart, joints, and nervous system and is capable of attachment to diverse cell types. Among the host components recognized by this spirochete are fibronectin and glycosaminoglycans (GAGs). Three surface-localized GAG-binding bacterial ligands, Bgp, DbpA, and DbpB, have been previously identified, but recent studies suggested that at least one additional GAG-binding ligand is expressed on the spirochetal surface when the spirochete is adapted to the mammalian host environment. BBK32 is a surface lipoprotein that is produced during infection and that has been shown to bind to fibronectin. In this study, we show that, when BBK32 was produced from a shuttle vector in an otherwise nonadherent high-passage B. burgdorferi strain, the protein localized on the bacterial surface and conferred attachment to fibronectin and to mammalian cell monolayers. In addition, the high-passage strain producing BBK32 bound to purified preparations of the GAGs dermatan sulfate and heparin, as well as to these GAGs on the surfaces of cultured mammalian cells. Recombinant BBK32 recognized purified heparin, indicating that the bacterial attachment to GAGs was due to direct binding by BBK32. This GAG-binding activity of BBK32 is apparently independent of fibronectin recognition, because exogenous heparin had no effect on BBK32-mediated bacterial binding to fibronectin.
Figures
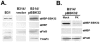


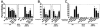
References
-
- Cadavid, D., V. Bundoc, and A. G. Barbour. 1993. Experimental infection of the mouse brain by a relapsing fever Borrelia species: a molecular analysis. J. Infect. Dis. 168:143-151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

