Identification and characterization of a novel bacterial virulence factor that shares homology with mammalian Toll/interleukin-1 receptor family proteins
- PMID: 16369016
- PMCID: PMC1346628
- DOI: 10.1128/IAI.74.1.594-601.2006
Identification and characterization of a novel bacterial virulence factor that shares homology with mammalian Toll/interleukin-1 receptor family proteins
Abstract
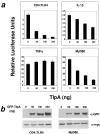
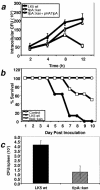
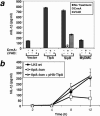
Many important bacterial virulence factors act as mimics of mammalian proteins to subvert normal host cell processes. To identify bacterial protein mimics of components of the innate immune signaling pathway, we searched the bacterial genome database for proteins with homology to the Toll/interleukin-1 receptor (TIR) domain of the mammalian Toll-like receptors (TLRs) and their adaptor proteins. A previously uncharacterized gene, which we have named tlpA (for TIR-like protein A), was identified in the Salmonella enterica serovar Enteritidis genome that is predicted to encode a protein resembling mammalian TIR domains, We show that overexpression of TlpA in mammalian cells suppresses the ability of mammalian TIR-containing proteins TLR4, IL-1 receptor, and MyD88 to induce the transactivation and DNA-binding activities of NF-kappaB, a downstream target of the TIR signaling pathway. In addition, TlpA mimics the previously characterized Salmonella virulence factor SipB in its ability to induce activation of caspase-1 in a mammalian cell transfection model. Disruption of the chromosomal tlpA gene rendered a virulent serovar Enteritidis strain defective in intracellular survival and IL-1beta secretion in a cell culture infection model using human THP1 macrophages. Bacteria with disrupted tlpA also displayed reduced lethality in mice, further confirming an important role for this factor in pathogenesis. Taken together, our findings demonstrate that the bacterial TIR-like protein TlpA is a novel prokaryotic modulator of NF-kappaB activity and IL-1beta secretion that contributes to serovar Enteritidis virulence.
Figures

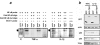
References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
-
- Athman, R., and D. Philpott. 2004. Innate immunity via Toll-like receptors and Nod proteins. Curr. Opin. Microbiol. 7:25-32. - PubMed
-
- Beutler, B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257-263. - PubMed
-
- Beutler, B., K. Hoebe, X. Du, and R. J. Ulevitch. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74:479-485. - PubMed
-
- Collier-Hyams, L. S., H. Zeng, J. Sun, A. D. Tomlinson, Z. Q. Bao, H. Chen, J. L. Madara, K. Orth, and A. S. Neish. 2002. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, antiapoptotic NF-κB pathway. J. Immunol. 169:2846-2850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

