Fusobacterium nucleatum transports noninvasive Streptococcus cristatus into human epithelial cells
- PMID: 16369022
- PMCID: PMC1346643
- DOI: 10.1128/IAI.74.1.654-662.2006
Fusobacterium nucleatum transports noninvasive Streptococcus cristatus into human epithelial cells
Erratum in
- Infect Immun. 2006 Dec;74(12):7045
Abstract
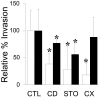
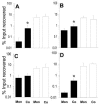
Analysis of human buccal epithelial cells frequently reveals an intracellular polymicrobial consortium of bacteria. Although several oral bacteria have been demonstrated to invade cultured epithelial cells, several others appear unable to internalize. We hypothesized that normally noninvasive bacteria may gain entry into epithelial cells via adhesion to invasive bacteria. Fusobacterium nucleatum is capable of binding to and invading oral epithelial cells. By contrast, Streptococcus cristatus binds weakly to host cells and is not internalized. F. nucleatum and S. cristatus coaggregate strongly via an arginine-sensitive interaction. Coincubation of KB or TERT-2 epithelial cells with equal numbers of F. nucleatum and S. cristatus bacteria led to significantly increased numbers of adherent and internalized streptococci. F. nucleatum also promoted invasion of KB cells by other oral streptococci and Actinomyces naeslundii. Dissection of fusobacterial or streptococcal adhesive interactions by using sugars, amino acids, or antibodies demonstrated that this phenomenon is due to direct attachment of S. cristatus to adherent and invading F. nucleatum. Inhibition of F. nucleatum host cell attachment and invasion with galactose, or fusobacterial-streptococcal coaggregation by the arginine homologue l-canavanine, abrogated the increased S. cristatus adhesion to, and invasion of, host cells. In addition, polyclonal antibodies to F. nucleatum, which inhibited fusobacterial attachment to both KB cells and S. cristatus, significantly decreased invasion by both species. Similar decreases were obtained when epithelial cells were pretreated with cytochalasin D, staurosporine, or cycloheximide. These studies indicate that F. nucleatum may facilitate the colonization of epithelial cells by bacteria unable to adhere or invade directly.
Figures






Similar articles
-
Streptococcus cristatus attenuates Fusobacterium nucleatum-induced interleukin-8 expression in oral epithelial cells.J Periodontal Res. 2008 Aug;43(4):408-16. doi: 10.1111/j.1600-0765.2007.01057.x. J Periodontal Res. 2008. PMID: 18942189
-
Streptococcus cristatus modulates the Fusobacterium nucleatum-induced epithelial interleukin-8 response through the nuclear factor-kappa B pathway.J Periodontal Res. 2011 Oct;46(5):558-67. doi: 10.1111/j.1600-0765.2011.01373.x. Epub 2011 Apr 26. J Periodontal Res. 2011. PMID: 21521225
-
Association of a high-molecular weight arginine-binding protein of Fusobacterium nucleatum ATCC 10953 with adhesion to secretory immunoglobulin A and coaggregation with Streptococcus cristatus.Oral Microbiol Immunol. 2007 Aug;22(4):217-24. doi: 10.1111/j.1399-302X.2006.00343.x. Oral Microbiol Immunol. 2007. PMID: 17600532
-
Fusobacterium nucleatum - symbiont, opportunist and oncobacterium.Nat Rev Microbiol. 2019 Mar;17(3):156-166. doi: 10.1038/s41579-018-0129-6. Nat Rev Microbiol. 2019. PMID: 30546113 Free PMC article. Review.
-
Fusobacterium nucleatum and cancer.Periodontol 2000. 2022 Jun;89(1):166-180. doi: 10.1111/prd.12426. Epub 2022 Mar 4. Periodontol 2000. 2022. PMID: 35244982 Free PMC article. Review.
Cited by
-
Biofilm and Cancer: Interactions and Future Directions for Cancer Therapy.Int J Mol Sci. 2023 Aug 16;24(16):12836. doi: 10.3390/ijms241612836. Int J Mol Sci. 2023. PMID: 37629016 Free PMC article. Review.
-
Potential Role of Biofilm Formation in the Development of Digestive Tract Cancer With Special Reference to Helicobacter pylori Infection.Front Microbiol. 2019 Apr 29;10:846. doi: 10.3389/fmicb.2019.00846. eCollection 2019. Front Microbiol. 2019. PMID: 31110496 Free PMC article.
-
Oral microbiome and health.AIMS Microbiol. 2018 Jan 12;4(1):42-66. doi: 10.3934/microbiol.2018.1.42. eCollection 2018. AIMS Microbiol. 2018. PMID: 31294203 Free PMC article. Review.
-
Periodontal pathogens and cancer development.Periodontol 2000. 2024 Oct;96(1):112-149. doi: 10.1111/prd.12590. Epub 2024 Jul 4. Periodontol 2000. 2024. PMID: 38965193 Free PMC article. Review.
-
Streptococcus cristatus attenuates Fusobacterium nucleatum-induced cytokine expression by influencing pathways converging on nuclear factor-κB.Mol Oral Microbiol. 2011 Apr;26(2):150-63. doi: 10.1111/j.2041-1014.2010.00600.x. Epub 2011 Jan 27. Mol Oral Microbiol. 2011. PMID: 21375705 Free PMC article.
References
-
- Babu, J. P., J. W. Dean, and M. J. Pabst. 1995. Attachment of Fusobacterium nucleatum to fibronectin immobilized on gingival epithelial cells or glass coverslips. J. Periodontol. 66:285-290. - PubMed
-
- Carranza, F. A, Jr., R. Saglie, M. G. Newman, and P. L. Valentin. 1983. Scanning and transmission electron microscopic study of tissue-invading microorganisms in localized juvenile periodontitis. J. Periodontol. 54:598-617. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

