CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium
- PMID: 16369024
- PMCID: PMC1346620
- DOI: 10.1128/IAI.74.1.673-681.2006
CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium
Abstract
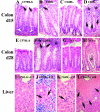
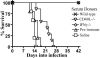
Citrobacter rodentium causes an attaching and effacing infection of the mouse colon. Surprisingly, protective adaptive immunity against this mucosal pathogen requires a systemic T-cell-dependent antibody response. To define CD4+ T-cell effector functions promoting this systemic defense of infected epithelial surfaces, studies were undertaken in weaning-age mice lacking costimulatory molecules CD28 or CD40L or cytokines gamma interferon (IFN-gamma) or interleukin-4 (IL-4). Adoptive transfer of CD4+ T cells from wild-type, CD28(-/-), CD40L(-/-), or IFN-gamma(-/-) donors to CD4(-/-) recipients delineated functions of these CD4+ T-cell-expressed molecules on the outcome of infection. Wild-type and IL-4(-/-) mice successfully resolved infection, while 70% of IFN-gamma(-/-) mice survived. In contrast, all CD28(-/-) mice succumbed during acute infection. While fewer than half of CD40L(-/-) mice succumbed acutely, surviving mice failed to clear infection, resulting in progressive mucosal destruction, polymicrobial sepsis, and death 1 to 2 weeks later than in CD28(-/-) mice. Downstream of CD28-mediated effects, CD4+ T-cell-expressed CD40L proved essential for generating acute pathogen-specific immunoglobulin M (IgM) and early IgG, which reduced pathogen burdens. However, deficiency of CD4+ T-cell-expressed IFN-gamma did not adversely impact survival or development of protective antibody in adoptively transferred CD4(-/-) recipients, though it impacted Th1 antibody responses. These findings demonstrate that CD4+ T-cell-expressed CD40L promotes the rapid production of protective systemic antibody during acute infection, while deficiencies of IL-4 or of CD4+ T-cell-expressed IFN-gamma can be overcome. These findings have important implications for understanding the role of T-helper-cell responses during infections involving mucosal surfaces.
Figures




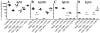

References
-
- Bertram, E. M., A. Tafuri, A. Shahinian, V. S. Chan, L. Hunziker, M. Recher, P. S. Ohashi, T. W. Mak, and T. H. Watts. 2002. Role of ICOS versus CD28 in antiviral immunity. Eur. J. Immunol. 32:3376-3385. - PubMed
-
- Bry, L., and M. B. Brenner. 2004. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 172:433-441. - PubMed
-
- Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. - PubMed
-
- Deenick, E. K., J. Hasbold, and P. D. Hodgkin. 1999. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J. Immunol. 163:4707-4714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

