A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity
- PMID: 16369026
- PMCID: PMC1346682
- DOI: 10.1128/IAI.74.1.694-702.2006
A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity
Abstract
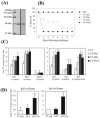
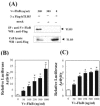
Flagellin, the structural component of flagellar filament in various locomotive bacteria, is the ligand for Toll-like receptor 5 (TLR5) of host cells. TLR stimulation by various pathogen-associated molecular patterns leads to activation of innate and subsequent adaptive immune responses. Therefore, TLR ligands are considered attractive adjuvant candidates in vaccine development. In this study, we show the highly potent mucosal adjuvant activity of a Vibrio vulnificus major flagellin (FlaB). Using an intranasal immunization mouse model, we observed that coadministration of the flagellin with tetanus toxoid (TT) induced significantly enhanced TT-specific immunoglobulin A (IgA) responses in both mucosal and systemic compartments and IgG responses in the systemic compartment. The mice immunized with TT plus FlaB were completely protected from systemic challenge with a 200x minimum lethal dose of tetanus toxin. Radiolabeled FlaB administered into the nasal cavity readily reached the cervical lymph nodes and systemic circulation. FlaB bound directly to human TLR5 expressed on cultured epithelial cells and consequently induced NF-kappaB and interleukin-8 activation. Intranasally administered FlaB colocalized with CD11c as patches in putative dendritic cells and caused an increase in the number of TLR5-expressing cells in cervical lymph nodes. These results indicate that flagellin would serve as an efficacious mucosal adjuvant inducing protective immune responses through TLR5 activation.
Figures

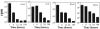


References
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Akira, S., and H, Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. - PubMed
-
- Applequist, S. E., R. P. Wallin, and H. G. Ljunggren. 2002. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int. Immunol. 14:1065-1074. - PubMed
-
- Boyaka, P. N., M. Ohmura, K. Fujihashi, T. Koga, M. Yamamoto, M. N. Kweon, Y. Takeda, R. J. Jackson, H. Kiyono, Y. Yuki, and J. R. McGhee. 2003. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. J. Immunol. 170:454-462. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

