Novel three-dimensional organoid model for evaluation of the interaction of uropathogenic Escherichia coli with terminally differentiated human urothelial cells
- PMID: 16369034
- PMCID: PMC1346604
- DOI: 10.1128/IAI.74.1.750-757.2006
Novel three-dimensional organoid model for evaluation of the interaction of uropathogenic Escherichia coli with terminally differentiated human urothelial cells
Abstract
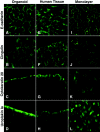
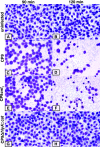
Human bladder 5637 cells cultivated under microgravity conditions formed organoids that displayed characteristics of in vivo tissue-specific differentiation. Uropathogenic Escherichia coli (UPEC) strain CP9 colonized and penetrated the organoids and induced alpha-hemolysin-mediated exfoliation of uroepithelial cells. We propose these uro-organoids as models that simulate the interactions between UPEC and terminally differentiated human urothelium.
Figures




Similar articles
-
Differentiation-induced uroplakin III expression promotes urothelial cell death in response to uropathogenic E. coli.Microbes Infect. 2009 Jan;11(1):57-65. doi: 10.1016/j.micinf.2008.10.008. Epub 2008 Nov 1. Microbes Infect. 2009. PMID: 19007907 Free PMC article.
-
Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis.Infect Immun. 2006 Sep;74(9):5106-13. doi: 10.1128/IAI.00376-06. Infect Immun. 2006. PMID: 16926402 Free PMC article.
-
Enhanced uropathogenic Escherichia coli-induced infection in uroepithelial cells by sugar through TLR-4 and JAK/STAT1 signaling pathways.J Microbiol Immunol Infect. 2021 Apr;54(2):193-205. doi: 10.1016/j.jmii.2019.05.008. Epub 2019 Jun 19. J Microbiol Immunol Infect. 2021. PMID: 31296484
-
Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney.Kidney Int. 2007 Jul;72(1):19-25. doi: 10.1038/sj.ki.5002230. Epub 2007 Mar 28. Kidney Int. 2007. PMID: 17396114 Review.
-
Virulence factors of uropathogenic E. coli and their interaction with the host.Adv Microb Physiol. 2014;65:337-72. doi: 10.1016/bs.ampbs.2014.08.006. Epub 2014 Nov 4. Adv Microb Physiol. 2014. PMID: 25476769 Review.
Cited by
-
3D Tumor Models in Urology.Int J Mol Sci. 2023 Mar 25;24(7):6232. doi: 10.3390/ijms24076232. Int J Mol Sci. 2023. PMID: 37047203 Free PMC article. Review.
-
Strengths and Limitations of Model Systems for the Study of Urinary Tract Infections and Related Pathologies.Microbiol Mol Biol Rev. 2016 Mar 2;80(2):351-67. doi: 10.1128/MMBR.00067-15. Print 2016 Jun. Microbiol Mol Biol Rev. 2016. PMID: 26935136 Free PMC article. Review.
-
Studying host-pathogen interactions in 3-D: organotypic models for infectious disease and drug development.J Neuroimmune Pharmacol. 2007 Mar;2(1):26-31. doi: 10.1007/s11481-006-9047-x. Epub 2006 Dec 6. J Neuroimmune Pharmacol. 2007. PMID: 18040823 Review.
-
Three-Dimensional Cell Culture Models to Study Respiratory Virus Infections Including COVID-19.Biomimetics (Basel). 2021 Dec 25;7(1):3. doi: 10.3390/biomimetics7010003. Biomimetics (Basel). 2021. PMID: 35076456 Free PMC article. Review.
-
Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli.Infect Immun. 2009 Jul;77(7):2762-72. doi: 10.1128/IAI.00323-09. Epub 2009 May 18. Infect Immun. 2009. PMID: 19451249 Free PMC article.
References
-
- Blum, G., V. Falbo, A. Caprioli, and J. Hacker. 1995. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and α-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol. Lett. 126:189-195. - PubMed
-
- Duncan, M. J., G. Li, J. S. Shin, J. L. Carson, and S. N. Abraham. 2004. Bacterial penetration of bladder epithelium through lipid rafts. J. Biol. Chem. 279:18944-18951. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

