Omalizumab: a monoclonal anti-IgE antibody
- PMID: 16369332
- PMCID: PMC1681435
Omalizumab: a monoclonal anti-IgE antibody
Abstract
Objectives: To describe allergic asthma and allergic rhinitis pathophysiology and review the pharmacologic, pharmacokinetic, pharmacodynamic, efficacy, and safety data for omalizumab.
Methods: MEDLINE, In-Process & Other Non-Indexed Citations, and EMBASE Drugs & Pharmacology were searched using olizumab, omalizumab, E25, rhuMAb-E25, and anti-IgE. Combinations of rhinitis, asthma, and IgE captured information on disease pathophysiology.
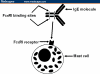
Results: Omalizumab is a monoclonal antibody targeting the high-affinity receptor binding site on human immunoglobulin (Ig)E. Bound IgE is not available for basophil binding, degranulation is attenuated, and allergic symptoms are reduced. In asthma trials, omalizumab reduced inhaled corticosteroid and rescue medication requirements and improved asthma control and asthma quality of life in moderate to severe allergic asthmatics with disease poorly controlled by inhaled corticosteroids. In trials of patients with poorly controlled moderate to severe seasonal allergic rhinitis (SAR), omalizumab reduced the severity of exacerbations and rescue medication use, and improved rhinitis-related quality of life. Benefits were also observed in trials utilizing combinations of immunotherapy and omalizumab for SAR and in trials of perennial allergic rhinitis (PAR). Omalizumab has been well tolerated. Although malignant neoplasms have been observed in treated patients, they were likely not related to omalizumab therapy.
Conclusions: Omalizumab has demonstrated efficacy in children, adults, and adolescents with uncontrolled moderate to severe allergic asthma and allergic rhinitis. Long-term safety beyond 52 weeks needs continued evaluation.
Figures

References
-
- Weiss KB, Sullivan SD, Lyttle CS. Trends in the cost of illness for asthma in the United States, 1985–1994. J Allergy Clin Immunol. 2000;106:493–499. - PubMed
-
- Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma — United States, 1980–1999. MMWR Surveill Summ. 2002;51(SS01):1–13. - PubMed
-
- Ford ES, Mannino DM, Homa DM, et al. Self-reported asthma and health related quality of life. Findings from the Behavioral Risk Factor Surveillance System. Chest. 2003;123:119–127. - PubMed
-
- Fineman SM. The burden of allergic rhinitis: beyond dollars and cents. Ann Allergy Asthma Immunol. 2002;88(Suppl):2–7. - PubMed
-
- Baroody FM. Allergic rhinitis: broader disease effects and implications for management. Otolaryngol Head Neck Surg. 2003;128:616–631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

