Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis
- PMID: 16369727
- PMCID: PMC3444246
- DOI: 10.1007/s00395-005-0581-0
Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis
Abstract
Objective: Pressure-overload hypertrophy is associated with decreased capillary density in myocardium resulting in impaired substrate delivery. Treatment of hypertrophied hearts with vascular endothelial growth factor (VEGF) induces angiogenesis. Since angiogenesis is associated with extracellular matrix degradation, we sought to determine whether VEGF induced angiogenesis in hypertrophy required matrix metalloproteinases (MMP) activation.
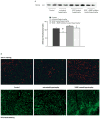
Methods: Newborn rabbits underwent aortic banding. Progression of hypertrophy (mass-to-volume (M/V) ratio) and mid-wall contractility index was monitored by echocardiography. At 4 and 6 weeks, VEGF (2 microg/kg), vehicle or VEGF combined with GM6001 (5 mg/kg), a MMP inhibitor, was administered intrapericardially. CD-31 (indicator of angiogenesis), MMP-2, MT1-MMP and TIMPs (endogenous MMP inhibitors) expression were measured by immunoblotting. MMP-2 activity was determined by gelatin zymography.
Results: Untreated hypertrophied hearts progressed to ventricular dilatation at 7 wks (M/V ratio: 0.75 +/- 0.07), but compensatory hypertrophy was maintained with VEGF (0.91 +/- 0.07; p < 0.05). LV contractility declined in untreated hearts from -0.41 +/- 0.9 (5 wks) to -0.73 +/- 0.5 (7 wks; p < 0.05) but remained normal with VEGF (+1.61 +/- 0.6 vs. +0.47 +/- 0.2). MMP-2 expression and activity were significantly elevated in VEGF treated hypertrophied hearts (p < 0.05) and were blocked by concomitant administration of GM6001. VEGF induced neovascularization was inhibited by addition of GM6001. MT1-MMP showed a trend to higher levels in VEGF treated hearts. TIMPs were unchanged in all three groups.
Conclusions: Exogenous VEGF and resultant MMP-2 activation leads to increased capillary formation in severe hypertrophy, preventing progression to ventricular dilation and dysfunction. VEGF and the associated MMP-2 activation play an important and potentially therapeutic role in vascular remodeling of hypertrophied hearts.
Figures



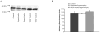

References
-
- Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89 (5):2183–2189. - PubMed
-
- Braunhut SJ, Moses MA. Retinoids modulate endothelial cell production of matrix-degrading proteases and tissue inhibitors of metalloproteinases (TIMP) J Biol Chem. 1994;269:13472–13479. - PubMed
-
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. - PubMed
-
- Coker ML, Doscher MA, Thomas CV, Galis ZS, Spinale FG. Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am J Physiol. 1999;277:H777–H787. - PubMed
-
- Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

