Gialpha and Gbeta subunits both define selectivity of G protein activation by alpha2-adrenergic receptors
- PMID: 16371464
- PMCID: PMC1325004
- DOI: 10.1073/pnas.0509763102
Gialpha and Gbeta subunits both define selectivity of G protein activation by alpha2-adrenergic receptors
Abstract
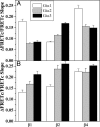
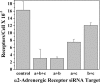
Previous studies of the specificity of receptor interactions with G protein subunits in living cells have relied on measurements of second messengers or other downstream responses. We have examined the selectivity of interactions between alpha2-adrenergic receptors (alpha2R) and various combinations of Gialpha and Gbeta subunit isoforms by measuring changes in FRET between Gialpha-yellow fluorescent protein and cyan fluorescent protein-Gbeta chimeras in HeLa cells. All combinations of Gialpha1, -2, or -3 with Gbeta1, -2, or -4 were activated to some degree by endogenous alpha2Rs as judged by agonist-dependent decreases in FRET. The degree of G protein activation is determined by the combination of Gialpha and Gbeta subunits rather than by the identity of an individual subunit. RT-PCR analysis and small interfering RNA knockdown of alpha2R subtypes, followed by quantification of radiolabeled antagonist binding, demonstrated that HeLa cells express alpha2a- and alpha2b-adrenergic receptor isoforms in a 2:1 ratio. Increasing receptor number by overexpression of the alpha2aR subtype minimized the differences among coupling preferences for Gialpha and Gbeta isoforms. The molecular properties of each Gialpha, Gbeta, and alpha2-adrenergic receptor subtype influence signaling efficiency for the alpha2-adrenergic receptor-mediated signaling pathway.
Figures





References
-
- Kleuss, C., Scherubl, H., Hescheler, J., Schultz, G. & Wittig, B. (1993) Science 259, 832–834. - PubMed
-
- Kleuss, C., Scherubl, H., Hescheler, J., Schultz, G. & Wittig, B. (1992) Nature 358, 424–426. - PubMed
-
- Kleuss, C., Hescheler, J., Ewel, C., Rosenthal, W., Schultz, G. & Wittig, B. (1991) Nature 353, 43–48. - PubMed
-
- Albert, P. R. & Robillard, L. (2002) Cell Signal. 14, 407–418. - PubMed
-
- Robishaw, J. D. & Berlot, C. H. (2004) Curr. Opin. Cell Biol. 16, 206–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

