Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex
- PMID: 16371475
- PMCID: PMC1324977
- DOI: 10.1073/pnas.0504909102
Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex
Abstract
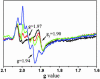
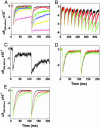
Details are presented of the structural analysis of the cytochrome b(6)f complex from the thermophilic cyanobacterium, Mastigocladus laminosus, in the presence of the electrochemically positive (p)-side quinone analogue inhibitor, 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB). One DBMIB binding site was found. This site is peripheral to the quinone binding space defined by the binding sites of other p-side inhibitors previously resolved in cytochrome bc(1)/b(6)f complexes. This high-affinity site resides in a p-side interfacial niche bounded by cytochrome f, subunit IV, and cytochrome b(6), is close (8 A) to the p-side heme b, but distant (19 A) from the [2Fe-2S] cluster. No significant electron density associated with the DBMIB was found elsewhere in the structure. However, the site at which DBMIB can inhibit light-induced redox turnover is within a few A of the [2Fe-2S] cluster, as shown by the absence of inhibition in mutants of Synechococcus sp. PCC 7002 at iron sulfur protein-Leu-111 near the cluster. The ability of a minimum amount of initially oxidized DBMIB to inhibit turnover of WT complex after a second light flash implies that there is a light-activated movement of DBMIB from the distal peripheral site to an inhibitory site proximal to the [2Fe-2S] cluster. Together with the necessary passage of quinone/quinol through the small Q(p) portal in the complex, it is seen that transmembrane traffic of quinone-like molecules through the core of cytochrome bc complexes can be labyrinthine.
Figures
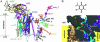


References
-
- Blankenship, R. E. (2002) Molecular Mechanisms of Photosynthesis (Blackwell, Malden, MA), pp. 124–156.
-
- Kurisu, G., Zhang, H., Smith, J. L. & Cramer, W. A. (2003) Science 302, 1009–1014. - PubMed
-
- Stroebel, D., Choquet, Y., Popot, J. L. & Picot, D. (2003) Nature 426, 413–418. - PubMed
-
- Xia, D., Yu, C. A., Kim, H., Xia, J. Z., Kachurin, A. M., Zhang, L., Yu, L. & Deisenhofer, J. (1997) Science 277, 60–66. - PubMed
-
- Zhang, Z., Huang, L., Shulmeister, V. M., Chi, Y. I., Kim, K. K., Hung, L. W., Crofts, A. R., Berry, E. A. & Kim, S. H. (1998) Nature 392, 677–684. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

