Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells
- PMID: 16371504
- PMCID: PMC1382315
- DOI: 10.1091/mbc.e05-10-0927
Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells
Abstract
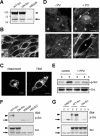
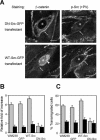
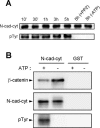
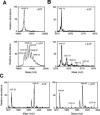
N-cadherin is recruited to the heterotypic contact during transendothelial migration of melanoma cells in a coculture system with tumor cells seeded on top of a monolayer of endothelial cells. However, beta-catenin dissociates from N-cadherin and redistributes to the nucleus of transmigrating melanoma cells to activate gene transcription. In this report, we demonstrate that Src becomes activated at the heterotypic contact between the transmigrating melanoma cell and neighboring endothelial cells. Src activation shows close temporal correlation with tyrosine phosphorylation of N-cadherin. Expression of a dominant-negative Src in melanoma cells blocks N-cadherin phosphorylation, beta-catenin dissociation, and nuclear translocation in transmigrating cells, consistent with the involvement of Src family kinases. In in vitro binding assays, Src-mediated phosphorylation of the N-cadherin cytoplasmic domain results in a significant reduction in beta-catenin binding. Although five phospho-tyrosine residues can be identified on the N-cadherin cytoplasmic domain by mass spectrometry, site-specific mutagenesis indicates that Tyr-860 is the critical amino acid involved in beta-catenin binding. Overexpression of N-cadherin carrying the Y860F mutation inhibits the transmigration of transfected cells across the endothelium. Together, the data suggest a novel role for tyrosine phosphorylation of N-cadherin by Src family kinases in the regulation of beta-catenin association during transendothelial migration of melanoma cells.
Figures

References
-
- Behrens, J., Vakaet, L., Friis, R., Winterhager, E., Van Roy, F., Mareel, M. M., and Birchmeier, W. (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-Src gene. J. Cell Biol. 120, 757–766. - PMC - PubMed
-
- Boyer, B., Bourgeois, Y., and Poupon, M. F. (2002). Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene 21, 2347–2356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

