Awake nonresectional lung volume reduction surgery
- PMID: 16371748
- PMCID: PMC1449981
- DOI: 10.1097/01.sla.0000182917.39534.2c
Awake nonresectional lung volume reduction surgery
Abstract
Objective: To assess the feasibility, safety, and early results of awake lung volume reduction surgery (LVRS) performed under thoracic epidural anesthesia by a new nonresectional technique.
Summary background data: So far, resectional LVRS under general anesthesia and one-lung ventilation is the more frequently used technique, but procedure-related morbidity has been considerable.
Methods: The study cohort included 12 patients undergoing unilateral awake LVRS. Evaluated parameters included technical feasibility and anesthesia satisfaction scored into 4 grades (from 1 = poor to 4 = excellent), global operating room time, and arterial carbon dioxide tension (PaCO2). In addition, 6-month changes in outcome measures, including forced expiratory volume in 1 second (FEV1), residual volume (RV), 6-minute walking test (SMWT), and dyspnea index were recorded. Perioperative and 6-month results were comparable with those of a control group undergoing unilateral resectional LVRS.
Results: Technical feasibility was excellent to satisfactory in 11 patients. One patient required conversion to one-lung ventilation. Differences between the awake and control group included global operating room time (90 +/- 17 minutes versus 145 +/- 19 minutes, P < 0.00001); PaCO2 24 hours after surgery (45 +/- 6 mm Hg versus 49 +/- 6 mm Hg, P = 0.02); and hospital stay (7.8 +/- 5 days versus 11.7 +/- 4 days, P = 0.02). Significant (P < 0.002) improvements occurred at 6 months in FEV1 (0.31 +/- 0.17 L), RV (-1.41 +/- 0.7 L), SMWT (73 +/- 25 m), and dyspnea index (-1.3 +/- 0.5) and were comparable with those of the control group.
Conclusions: In this study, awake nonresectional LVRS proved feasible and safe. This new modality was associated with a faster recovery and satisfactory 6-month outcome, which did not differ from that of resectional LVRS.
Figures

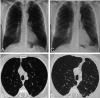
References
-
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. - PubMed
-
- Pompeo E, Marino M, Nofroni I, et al. Reduction pneumoplasty versus respiratory rehabilitation: a randomised trial. Ann Thorac Surg. 2000;70:948–954. - PubMed
-
- Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg. 2003;125:513–525. - PubMed
-
- National Emphysema Treatment Trial Research group. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med. 2003;348:2092–2102. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

